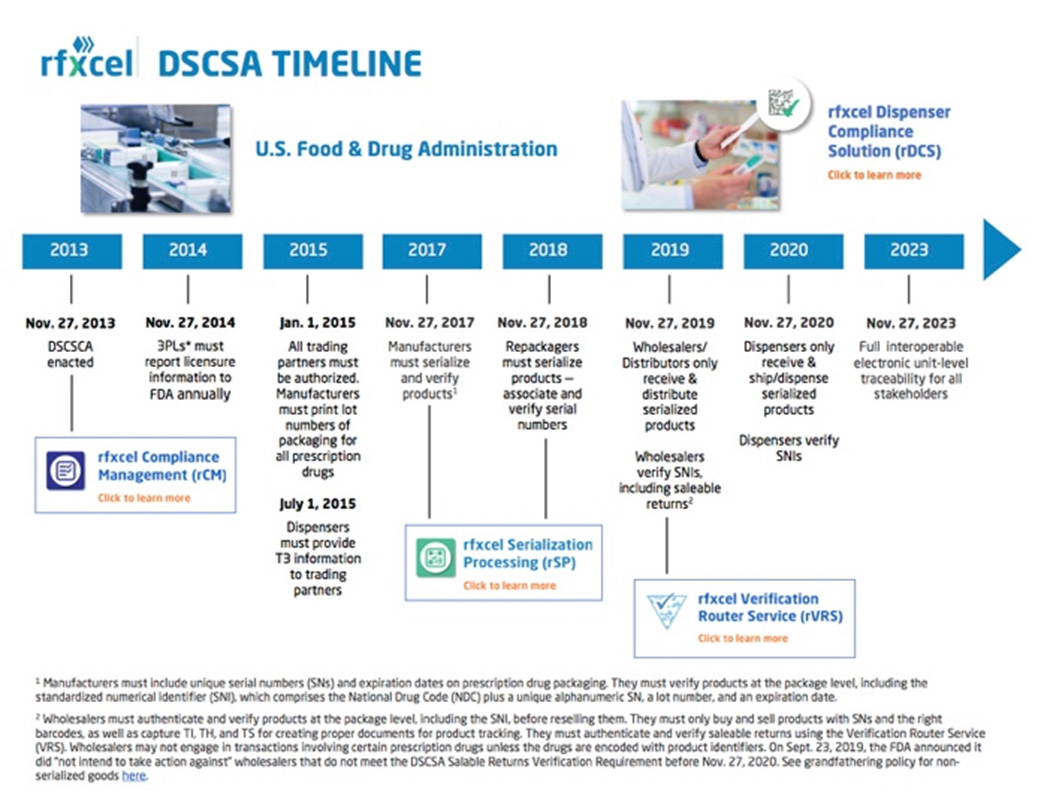
Dscsa Expiration Date Format . Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning. The dscsa, title ii of the. Yes, the serial number, lot number and expiration. Expiration date (product identifier) in human readable format for each package in an order? Expiration date of the product. Supply chain and security act (dscsa), which is scheduled to take full effect by nov. The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able to exchange the data electronically.
from rfxcel.com
The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able to exchange the data electronically. Expiration date of the product. Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. Expiration date (product identifier) in human readable format for each package in an order? The dscsa, title ii of the. Yes, the serial number, lot number and expiration. Supply chain and security act (dscsa), which is scheduled to take full effect by nov. Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning.
U.S. Drug Supply Chain Security Act (DSCSA)
Dscsa Expiration Date Format Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. Yes, the serial number, lot number and expiration. Expiration date (product identifier) in human readable format for each package in an order? Expiration date of the product. Supply chain and security act (dscsa), which is scheduled to take full effect by nov. The dscsa, title ii of the. Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able to exchange the data electronically.
From food.chemlinked.com
Malaysia Food Labeling Regulation ChemLinked Dscsa Expiration Date Format Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. Supply chain and security act (dscsa), which is scheduled to take full effect by nov. The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. Expiration date (product identifier) in human readable format for. Dscsa Expiration Date Format.
From rfxcel.com
U.S. Drug Supply Chain Security Act (DSCSA) Dscsa Expiration Date Format Supply chain and security act (dscsa), which is scheduled to take full effect by nov. The dscsa, title ii of the. Expiration date (product identifier) in human readable format for each package in an order? Yes, the serial number, lot number and expiration. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include. Dscsa Expiration Date Format.
From medpak.com
DSCSA Compliance 2024 Is Your Organization Ready? Dscsa Expiration Date Format The dscsa, title ii of the. Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. Expiration date (product identifier) in human readable format for each package in an order? Supply chain and security act (dscsa), which is scheduled to take full effect by nov. Yes, the serial number, lot number and. Dscsa Expiration Date Format.
From www.oc-i.org
Four Steps to DSCSA Stabilization in 2024 — Open Credentialing Initiative Dscsa Expiration Date Format Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning. Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be. Dscsa Expiration Date Format.
From www.youtube.com
Understanding the Dates on Food Labels Are Canned Goods Safe After the Dscsa Expiration Date Format The dscsa, title ii of the. Supply chain and security act (dscsa), which is scheduled to take full effect by nov. Yes, the serial number, lot number and expiration. The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies. Dscsa Expiration Date Format.
From knowleswellness.com
Medication Expiration Date Guidelines by FDA KnowlesWellness Dscsa Expiration Date Format Expiration date (product identifier) in human readable format for each package in an order? Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning. Supply chain and security act (dscsa), which is scheduled to take full effect by nov. Expiration date of the product. Yes, the serial number, lot number. Dscsa Expiration Date Format.
From www.healthcarepackaging.com
Key Concepts of the FDA’s Drug Supply Chain Security Act (DSCSA Dscsa Expiration Date Format The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. Expiration date of the product. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able to exchange the data electronically. Yes, the serial number, lot number and expiration. Expiration. Dscsa Expiration Date Format.
From www.reddit.com
What is the expiration date format, and when does it even expire? r Dscsa Expiration Date Format Supply chain and security act (dscsa), which is scheduled to take full effect by nov. Expiration date (product identifier) in human readable format for each package in an order? Yes, the serial number, lot number and expiration. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able. Dscsa Expiration Date Format.
From rfxcel.com
DSCSA Serialization Implementation and Compliance Guidelines 2023 Dscsa Expiration Date Format Expiration date of the product. Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning. Expiration date (product identifier) in human readable format for each package in an order? Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. The dscsa, title. Dscsa Expiration Date Format.
From www.oc-i.org
The DSCSA Deadline What It Means and How to Prepare — Open Dscsa Expiration Date Format Expiration date of the product. The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. Yes, the serial number, lot number and expiration. Expiration date (product identifier) in human readable format for each package in an order? The dscsa, title ii of the. As we approach the dscsa’s final implementation deadline (november. Dscsa Expiration Date Format.
From deacom.com
GS1128 Barcodes and Inventory Management ERP Dscsa Expiration Date Format Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. The dscsa, title ii of the. The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. Expiration date (product identifier) in human readable format for each package in an order? As we approach the. Dscsa Expiration Date Format.
From www.scandit.com
How smart devices help retail pharmacies meet DSCSA 2023 requirements Dscsa Expiration Date Format Supply chain and security act (dscsa), which is scheduled to take full effect by nov. Yes, the serial number, lot number and expiration. Expiration date of the product. The dscsa, title ii of the. Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning. Those dscsa requirements are scheduled to. Dscsa Expiration Date Format.
From www.drugtopics.com
The Path Forward Navigating the DSCSA Stabilization Policy Dscsa Expiration Date Format The dscsa, title ii of the. Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction. Dscsa Expiration Date Format.
From www.mlo-online.com
Establishing expiry date for clinical diagnostic reagents Medical Dscsa Expiration Date Format Yes, the serial number, lot number and expiration. Expiration date (product identifier) in human readable format for each package in an order? Supply chain and security act (dscsa), which is scheduled to take full effect by nov. Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning. Expiration date of. Dscsa Expiration Date Format.
From lighthouseai.com
DSCSA Requirements and Implementation A Solutions Map AI to Protect Dscsa Expiration Date Format Expiration date (product identifier) in human readable format for each package in an order? As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able to exchange the data electronically. The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial.. Dscsa Expiration Date Format.
From www.pharmacytimes.com
Unraveling the DSCSA Serialization Puzzle Implications for Drug Dscsa Expiration Date Format The dscsa, title ii of the. Expiration date of the product. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able to exchange the data electronically. Expiration date (product identifier) in human readable format for each package in an order? Those dscsa requirements are scheduled to change. Dscsa Expiration Date Format.
From pharmacyequipmentmarketplace.com
DSCSA Procedures RxMarketplace Dscsa Expiration Date Format Supply chain and security act (dscsa), which is scheduled to take full effect by nov. Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning. Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. The dscsa, title ii of the. Yes,. Dscsa Expiration Date Format.
From www.unitdosemedication.com
Unit Dose Relabeling HealthFirst Dscsa Expiration Date Format Yes, the serial number, lot number and expiration. The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. Supply chain and security act (dscsa), which is scheduled to take full effect by nov. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level. Dscsa Expiration Date Format.
From www.researchgate.net
Expirationdate inscriptions illustrating variable legibility, format Dscsa Expiration Date Format As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able to exchange the data electronically. Yes, the serial number, lot number and expiration. Supply chain and security act (dscsa), which is scheduled to take full effect by nov. Those dscsa requirements are scheduled to change on november. Dscsa Expiration Date Format.
From www.pppmag.com
Choosing a DSCSA Compliance Service September 2017 Pharmacy Dscsa Expiration Date Format Expiration date of the product. The dscsa, title ii of the. Expiration date (product identifier) in human readable format for each package in an order? The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. Yes, the serial number, lot number and expiration date will be required as part of the dscsa. Dscsa Expiration Date Format.
From lspedia.com
a DSCSA subject expert with LSPediA training, December 78 Dscsa Expiration Date Format Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning. The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. The dscsa, title ii of the. Expiration date (product identifier) in human readable format for each package in an order? Those dscsa. Dscsa Expiration Date Format.
From www.rxtrace.com
Global Differences In Expiration Date Encoding RxTrace Dscsa Expiration Date Format As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able to exchange the data electronically. Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning. The dscsa, title ii of the. The standardized numerical identifier, a component. Dscsa Expiration Date Format.
From www.healthcarepackaging.com
Serialization 101 Healthcare Packaging Dscsa Expiration Date Format Expiration date of the product. The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. The dscsa, title ii of the. Expiration date (product identifier) in human readable format for each package in an order? Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners. Dscsa Expiration Date Format.
From www.tracekey.com
DSCSA Requirements for 2023 tracekey solutions GmbH Dscsa Expiration Date Format Expiration date (product identifier) in human readable format for each package in an order? Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. Supply chain and security act (dscsa), which is scheduled to take full effect by nov. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies. Dscsa Expiration Date Format.
From info.docxellent.com
2023 Pharmaceutical Guidelines Ensuring DSCSA Compliance Dscsa Expiration Date Format Supply chain and security act (dscsa), which is scheduled to take full effect by nov. Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning. Expiration date of the product. The dscsa, title ii of the. Yes, the serial number, lot number and expiration. Expiration date (product identifier) in human. Dscsa Expiration Date Format.
From rfxcel.com
DSCSA Summary Highlights of the Drug Supply Chain Security Act Dscsa Expiration Date Format Supply chain and security act (dscsa), which is scheduled to take full effect by nov. The dscsa, title ii of the. Expiration date (product identifier) in human readable format for each package in an order? The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. Yes, the serial number, lot number and. Dscsa Expiration Date Format.
From www.oc-i.org
DSCSA for Dispensers and their Trading Partners Virtual Conference Dscsa Expiration Date Format As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able to exchange the data electronically. Yes, the serial number, lot number and expiration. The dscsa, title ii of the. Supply chain and security act (dscsa), which is scheduled to take full effect by nov. Yes, the serial. Dscsa Expiration Date Format.
From www.pharmaceuticalonline.com
Verification Of Serialized Drug Product — 60 Million Reasons Why Dscsa Expiration Date Format Expiration date of the product. Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able to exchange the data electronically. The dscsa, title ii of the. Yes, the serial. Dscsa Expiration Date Format.
From www.pharmamanufacturing.com
Prepare Now for DSCSA Serialization Pharma Manufacturing Dscsa Expiration Date Format Expiration date of the product. Yes, the serial number, lot number and expiration. The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. Expiration date (product identifier) in human readable format for each package in an order? Supply chain and security act (dscsa), which is scheduled to take full effect by nov.. Dscsa Expiration Date Format.
From info.docxellent.com
2023 Pharmaceutical Guidelines Ensuring DSCSA Compliance Dscsa Expiration Date Format The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able to exchange the data electronically. Supply chain and security act (dscsa), which is scheduled to take full effect by. Dscsa Expiration Date Format.
From www.tracekey.com
Understanding VRS The 2023 DSCSA requirements tracekey solutions GmbH Dscsa Expiration Date Format Expiration date (product identifier) in human readable format for each package in an order? Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning. Expiration date of the product. The dscsa, title ii of the. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected. Dscsa Expiration Date Format.
From www.enfamil.com
Expiration Dates, Lot Numbers & Batch Codes Enfamil Dscsa Expiration Date Format Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. The dscsa, title ii of the. As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able to exchange the data electronically. The standardized numerical identifier, a component of the. Dscsa Expiration Date Format.
From slideplayer.com
Enforcement Litigation and Compliance Washington, DC December 910 Dscsa Expiration Date Format Those dscsa requirements are scheduled to change on november 27, 2023, and will include requiring trading partners to. Expiration date of the product. Yes, the serial number, lot number and expiration. Expiration date (product identifier) in human readable format for each package in an order? As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected. Dscsa Expiration Date Format.
From www.drugtopics.com
DSCSA Keys to full compliance Dscsa Expiration Date Format Yes, the serial number, lot number and expiration date will be required as part of the dscsa transaction information beginning. Supply chain and security act (dscsa), which is scheduled to take full effect by nov. The standardized numerical identifier, a component of the product identifier, is comprised of the ndc and a serial. The dscsa, title ii of the. Yes,. Dscsa Expiration Date Format.
From www.rxtrace.com
Decoding The FDA's DSCSA Timeline RxTrace Dscsa Expiration Date Format As we approach the dscsa’s final implementation deadline (november 27th, 2023), pharmaceutical companies are expected to include unit level serial and be able to exchange the data electronically. Expiration date (product identifier) in human readable format for each package in an order? Yes, the serial number, lot number and expiration. Those dscsa requirements are scheduled to change on november 27,. Dscsa Expiration Date Format.