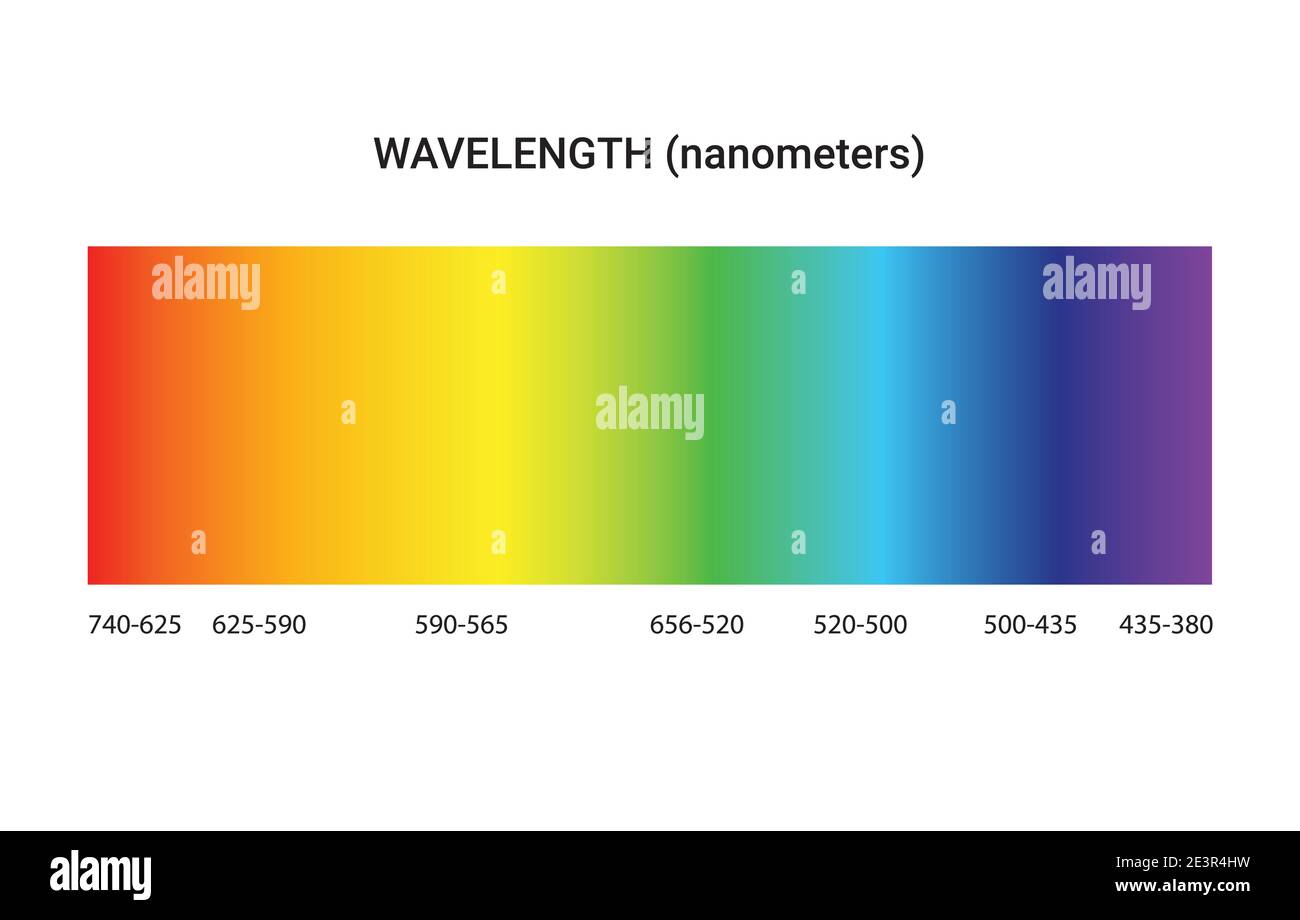
What Is Light Emission Spectrum . Absorption spectra are lit with dark bands; When hydrogen gas is placed. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. Emission spectra are dark with lit. Emission spectra of carbon, oxygen, nitrogen, and iron. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Each element emits a unique pattern of colors, or wavelengths, of visible light. Astronomers can analyze the pattern of light. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Every atomic element has a unique absorption and emission spectrum.
from www.alamy.com
When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission spectra are dark with lit. Every atomic element has a unique absorption and emission spectrum. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission spectra of carbon, oxygen, nitrogen, and iron. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Astronomers can analyze the pattern of light. Absorption spectra are lit with dark bands;
Emission spectrum hires stock photography and images Alamy
What Is Light Emission Spectrum Astronomers can analyze the pattern of light. Every atomic element has a unique absorption and emission spectrum. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. Each element emits a unique pattern of colors, or wavelengths, of visible light. Astronomers can analyze the pattern of light. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. When hydrogen gas is placed. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Absorption spectra are lit with dark bands; Emission spectra of carbon, oxygen, nitrogen, and iron. Emission spectra are dark with lit. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.
From newgradoptometry.com
Understanding Acuvue Contacts and Ultraviolet Light What Is Light Emission Spectrum Emission spectra are dark with lit. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. Astronomers can analyze the pattern of light. Each element emits a unique pattern of colors, or wavelengths, of visible light. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known. What Is Light Emission Spectrum.
From www.researchgate.net
Emission spectra of LED light sources. Download Scientific Diagram What Is Light Emission Spectrum The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Astronomers can analyze the pattern of light. Absorption spectra are lit with dark bands; Emission spectra of carbon, oxygen, nitrogen, and iron. Atoms of individual elements emit light at only specific wavelengths, producing a. What Is Light Emission Spectrum.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts What Is Light Emission Spectrum Every atomic element has a unique absorption and emission spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. When hydrogen gas is placed.. What Is Light Emission Spectrum.
From webbtelescope.org
Types of Spectra Continuous, Emission, and Absorption b What Is Light Emission Spectrum Emission spectra are dark with lit. Emission spectra of carbon, oxygen, nitrogen, and iron. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. When hydrogen gas is placed. Absorption spectra are lit with dark bands; Atoms of individual elements emit light at only specific wavelengths,. What Is Light Emission Spectrum.
From stock.adobe.com
Continuous spectrum, an emission spectrum that consists of continuum of What Is Light Emission Spectrum The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Every atomic element has a unique absorption and emission spectrum. Each element emits a unique pattern of colors, or wavelengths, of visible light. This spectrum of radiation emitted by electrons in the excited atoms. What Is Light Emission Spectrum.
From www.slideserve.com
PPT Properties of Light PowerPoint Presentation, free download ID What Is Light Emission Spectrum Emission spectra of carbon, oxygen, nitrogen, and iron. Each element emits a unique pattern of colors, or wavelengths, of visible light. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either. What Is Light Emission Spectrum.
From alevelphysics.co.uk
Energy Levels & Photon Emission A Level Physics Revision Notes What Is Light Emission Spectrum Absorption spectra are lit with dark bands; Every atomic element has a unique absorption and emission spectrum. Emission spectra are dark with lit. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. When hydrogen gas is placed. In chemistry, an emission spectrum refers to the range of wavelengths emitted by. What Is Light Emission Spectrum.
From www.researchgate.net
Emission spectra of different light sources (a) incandescent tungsten What Is Light Emission Spectrum Each element emits a unique pattern of colors, or wavelengths, of visible light. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Absorption spectra are lit with dark bands; When hydrogen gas is placed. Emission spectra of carbon, oxygen, nitrogen, and iron. Atoms. What Is Light Emission Spectrum.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra What Is Light Emission Spectrum In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Every atomic element has a unique absorption and emission spectrum. Each element emits a unique pattern of. What Is Light Emission Spectrum.
From www.slideserve.com
PPT Emission Spectrum Animation PowerPoint Presentation, free What Is Light Emission Spectrum In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. When hydrogen gas is placed. Astronomers can analyze the pattern of light. Emission spectra are dark with lit. Absorption spectra are lit with dark bands; Emission spectra of carbon, oxygen, nitrogen, and iron. This spectrum of. What Is Light Emission Spectrum.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum What Is Light Emission Spectrum Absorption spectra are lit with dark bands; Every atomic element has a unique absorption and emission spectrum. Emission spectra of carbon, oxygen, nitrogen, and iron. When hydrogen gas is placed. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Emission spectra are dark with lit.. What Is Light Emission Spectrum.
From spiff.rit.edu
Spectrographs and Spectra What Is Light Emission Spectrum Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Every atomic element has a unique absorption and emission spectrum. Absorption spectra are lit with dark bands;. What Is Light Emission Spectrum.
From www.freepik.com
Premium Vector Visible light diagram. Color spectrum What Is Light Emission Spectrum When hydrogen gas is placed. Every atomic element has a unique absorption and emission spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Emission spectra are dark with lit. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather. What Is Light Emission Spectrum.
From imagine.gsfc.nasa.gov
Spectra Introduction What Is Light Emission Spectrum Emission spectra of carbon, oxygen, nitrogen, and iron. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. The emission spectrum (or line spectrum) of a chemical. What Is Light Emission Spectrum.
From www.dreamstime.com
The Spectrum Vector Diagram Stock Vector Illustration What Is Light Emission Spectrum Each element emits a unique pattern of colors, or wavelengths, of visible light. Emission spectra of carbon, oxygen, nitrogen, and iron. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either. What Is Light Emission Spectrum.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ What Is Light Emission Spectrum Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. Astronomers can analyze the pattern of light. When hydrogen gas is placed. Absorption spectra are lit with dark bands; Each element emits a unique pattern of colors, or wavelengths, of visible light. The emission spectrum (or line spectrum) of a chemical. What Is Light Emission Spectrum.
From analytik.co.uk
Visible Light Spectrum Analytik Ltd What Is Light Emission Spectrum Absorption spectra are lit with dark bands; This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. When hydrogen gas is placed. Every atomic element has a unique absorption and emission spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by. What Is Light Emission Spectrum.
From studyposter.blogspot.com
How Is A Stars Emission Spectrum Used To Study Stars Study Poster What Is Light Emission Spectrum Emission spectra are dark with lit. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Emission spectra of carbon, oxygen, nitrogen, and iron. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Each element emits. What Is Light Emission Spectrum.
From waves.neocities.org
The Spectrum What Is Light Emission Spectrum This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission spectra are dark with lit. Emission spectra of carbon, oxygen, nitrogen, and iron. Absorption spectra are lit with dark bands; When hydrogen gas is placed. Every atomic element has a unique absorption and emission spectrum. In chemistry, an emission spectrum. What Is Light Emission Spectrum.
From geoengineering.global
Solar Radiation ManagementReflecting Sunlight to Cool the Climate What Is Light Emission Spectrum Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. Each element emits a unique pattern of colors, or wavelengths, of visible light. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. This spectrum. What Is Light Emission Spectrum.
From atmos.washington.edu
Radiation spectrum for the Sun and Earth. What Is Light Emission Spectrum Every atomic element has a unique absorption and emission spectrum. When hydrogen gas is placed. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Emission spectra of carbon, oxygen, nitrogen, and iron. Atoms of individual elements emit light at only specific wavelengths, producing a line. What Is Light Emission Spectrum.
From www.alamy.com
Emission spectrum hires stock photography and images Alamy What Is Light Emission Spectrum Every atomic element has a unique absorption and emission spectrum. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Absorption spectra are lit with dark bands; Each element emits a unique pattern of colors, or wavelengths, of visible light. Astronomers can analyze the pattern of light. When hydrogen gas is. What Is Light Emission Spectrum.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog What Is Light Emission Spectrum Emission spectra of carbon, oxygen, nitrogen, and iron. Emission spectra are dark with lit. Each element emits a unique pattern of colors, or wavelengths, of visible light. When hydrogen gas is placed. Every atomic element has a unique absorption and emission spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when. What Is Light Emission Spectrum.
From www.scientifica.uk.com
Fluorescence light sources A comparative guide What Is Light Emission Spectrum Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. Astronomers can analyze the pattern of light. Each element emits a unique pattern of colors, or wavelengths, of visible light. Every atomic element has a unique absorption and emission spectrum. The emission spectrum (or line spectrum) of a chemical element is. What Is Light Emission Spectrum.
From www.pinterest.com
Absorption spectrum of the elements spectroscopy Physics, Earth and What Is Light Emission Spectrum Astronomers can analyze the pattern of light. Emission spectra are dark with lit. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Every atomic element has a unique absorption and emission spectrum. This spectrum of radiation emitted by electrons in the excited atoms. What Is Light Emission Spectrum.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning What Is Light Emission Spectrum Emission spectra are dark with lit. Astronomers can analyze the pattern of light. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Absorption spectra are lit with dark bands; Emission spectra of carbon, oxygen, nitrogen, and iron. The emission spectrum (or line spectrum) of a chemical element is the unique. What Is Light Emission Spectrum.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts What Is Light Emission Spectrum Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. Emission spectra are dark with lit. Astronomers can analyze the pattern of light. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Absorption spectra are lit. What Is Light Emission Spectrum.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction What Is Light Emission Spectrum Each element emits a unique pattern of colors, or wavelengths, of visible light. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. When hydrogen gas is placed. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission. What Is Light Emission Spectrum.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations What Is Light Emission Spectrum In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Every atomic element has a unique absorption and emission spectrum. Atoms of individual elements emit light at. What Is Light Emission Spectrum.
From www.researchgate.net
Light emission spectrum from a gold tipgold surface junction taken at What Is Light Emission Spectrum Emission spectra are dark with lit. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Each element emits a. What Is Light Emission Spectrum.
From users.highland.edu
Atomic Spectra and Models of the Atom What Is Light Emission Spectrum Emission spectra are dark with lit. When hydrogen gas is placed. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. Each element emits a unique pattern of colors, or wavelengths, of visible light. Emission spectra of carbon, oxygen, nitrogen, and iron. This spectrum of radiation emitted by electrons in the. What Is Light Emission Spectrum.
From www.thoughtco.com
Visible Light Spectrum Overview and Chart What Is Light Emission Spectrum Absorption spectra are lit with dark bands; In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Each element emits. What Is Light Emission Spectrum.
From www.slideserve.com
PPT Light Emission PowerPoint Presentation, free download ID5593597 What Is Light Emission Spectrum Emission spectra are dark with lit. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. Absorption spectra are lit with dark bands; In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Astronomers can analyze the. What Is Light Emission Spectrum.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle What Is Light Emission Spectrum When hydrogen gas is placed. Each element emits a unique pattern of colors, or wavelengths, of visible light. Emission spectra are dark with lit. Absorption spectra are lit with dark bands; In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. This spectrum of radiation emitted. What Is Light Emission Spectrum.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room What Is Light Emission Spectrum Each element emits a unique pattern of colors, or wavelengths, of visible light. Astronomers can analyze the pattern of light. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. What Is Light Emission Spectrum.