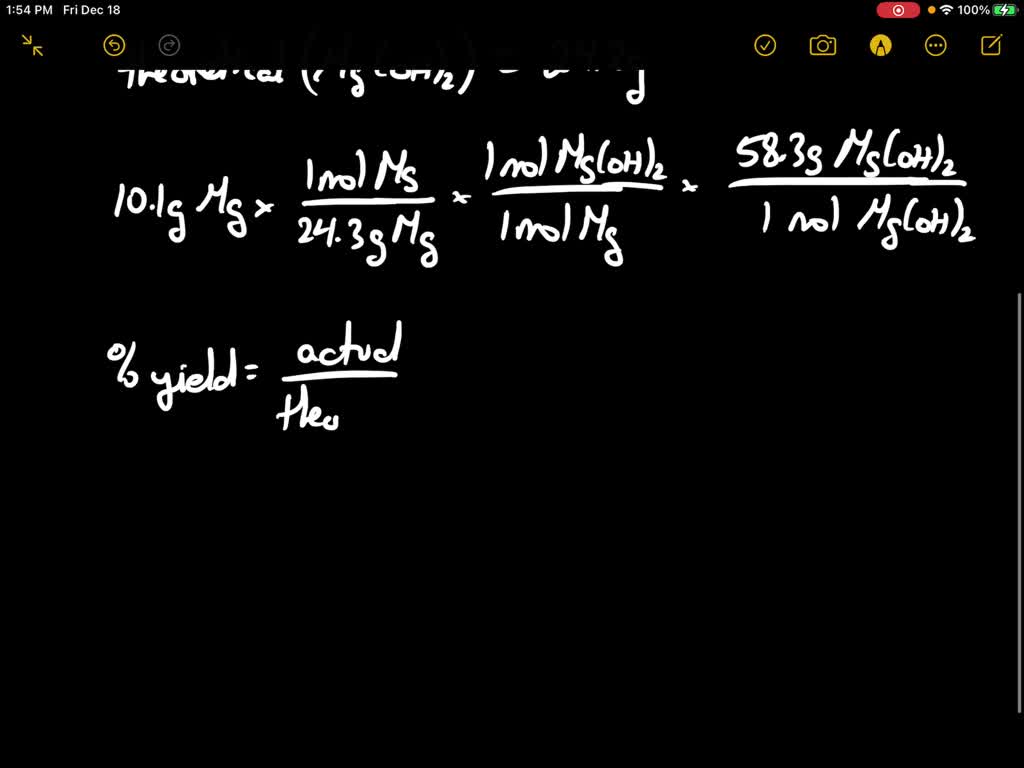Magnesium Hydroxide And Hydrogen Gas Formula . Molar masses of elements in reactants atomic mass. Be sure to indicate the state (s,l,g or aq) for each substance. M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. Magnesium + water → magnesium hydroxide + dihydrogen. Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. Mg(oh)2 + hcl = mgcl2 + h2o is a double displacement. The equation for the reactions of any of these metals would be: Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. Write a balanced equation for this reaction. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very.
from www.numerade.com
The equation for the reactions of any of these metals would be: X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. Molar masses of elements in reactants atomic mass. M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. Be sure to indicate the state (s,l,g or aq) for each substance.
Magnesium powder reacts with steam to form magnesium hydroxide and
Magnesium Hydroxide And Hydrogen Gas Formula The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. The equation for the reactions of any of these metals would be: M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Be sure to indicate the state (s,l,g or aq) for each substance. Write a balanced equation for this reaction. Mg(oh)2 + hcl = mgcl2 + h2o is a double displacement. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. Magnesium + water → magnesium hydroxide + dihydrogen. Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Molar masses of elements in reactants atomic mass.
From brainly.in
9.Write the chemical formula (i) Hydrochloric acid (ii) Magnesium Magnesium Hydroxide And Hydrogen Gas Formula Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. Write a balanced equation for this reaction. The equation for the reactions of any of these metals would be: The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. Magnesium burns. Magnesium Hydroxide And Hydrogen Gas Formula.
From lilynewsbranch.blogspot.com
Which Chemical Equation Shows the Dissociation of Magnesium Hydroxide Magnesium Hydroxide And Hydrogen Gas Formula M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. Molar masses of elements in reactants atomic mass. Magnesium + water → magnesium hydroxide + dihydrogen.. Magnesium Hydroxide And Hydrogen Gas Formula.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Hydroxide And Hydrogen Gas Formula Write a balanced equation for this reaction. M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. The equation for the reactions of any of these metals would be: X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. Magnesium + water → magnesium hydroxide + dihydrogen.. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.youtube.com
How to Draw the Lewis Dot Structure for Mg(OH)2 Magnesium hydroxide Magnesium Hydroxide And Hydrogen Gas Formula Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. The equation for the reactions of any of these metals would be: Write a balanced equation for this reaction. Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.youtube.com
How to Write the Formula for Magnesium hydroxide YouTube Magnesium Hydroxide And Hydrogen Gas Formula Be sure to indicate the state (s,l,g or aq) for each substance. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Write a balanced equation for this reaction. Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. The equation for the reactions of any of these metals would be: Molar. Magnesium Hydroxide And Hydrogen Gas Formula.
From brainly.in
the molecular formula of magnisium hydroxide by criss cross method Magnesium Hydroxide And Hydrogen Gas Formula Magnesium + water → magnesium hydroxide + dihydrogen. Write a balanced equation for this reaction. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Mg(oh)2 + hcl = mgcl2 + h2o is a double displacement.. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.youtube.com
H2SO4+Mg(OH)2=H2O+MgSO4 Balanced EquationSulphuric Acid+Magnesium Magnesium Hydroxide And Hydrogen Gas Formula Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Write a balanced equation for this reaction. Magnesium + water → magnesium hydroxide + dihydrogen. M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. Be sure to indicate. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.teachoo.com
[MCQ] Which statements is incorrect for magnesium metal? Teachoo Magnesium Hydroxide And Hydrogen Gas Formula The equation for the reactions of any of these metals would be: Mg(oh)2 + hcl = mgcl2 + h2o is a double displacement. X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. Write a balanced equation. Magnesium Hydroxide And Hydrogen Gas Formula.
From slideplayer.com
Ch. 7 Chemical Reactions ppt download Magnesium Hydroxide And Hydrogen Gas Formula X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Write a balanced equation for this reaction. M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. Be sure. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.numerade.com
SOLVED Solid magnesium hydroxide into gaseous water and Magnesium Hydroxide And Hydrogen Gas Formula Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. Magnesium + water → magnesium hydroxide + dihydrogen. Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. Molar masses of elements in reactants atomic mass. M. Magnesium Hydroxide And Hydrogen Gas Formula.
From ar.inspiredpencil.com
Magnesium Hydroxide Structure Magnesium Hydroxide And Hydrogen Gas Formula X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Mg(oh)2 + hcl = mgcl2 + h2o is a double displacement. Write a balanced equation for this reaction. Magnesium reacts with water to form magnesium hydroxide. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.youtube.com
How to write the formula for magnesium hydroxide YouTube Magnesium Hydroxide And Hydrogen Gas Formula Mg(oh)2 + hcl = mgcl2 + h2o is a double displacement. The equation for the reactions of any of these metals would be: Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Write a balanced. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.numerade.com
SOLVED'Write balanced equalion for the reaction _ Sodium hydroxide is Magnesium Hydroxide And Hydrogen Gas Formula Be sure to indicate the state (s,l,g or aq) for each substance. Molar masses of elements in reactants atomic mass. The equation for the reactions of any of these metals would be: \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.numerade.com
Metallic magnesium reacts with steam to produce solid magnesium Magnesium Hydroxide And Hydrogen Gas Formula M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. Write a balanced equation for this reaction. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Mg(oh)2 + hcl = mgcl2 + h2o is a double displacement. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. X (s). Magnesium Hydroxide And Hydrogen Gas Formula.
From www.numerade.com
SOLVEDWrite a balanced molecular equation and a net ionic equation for Magnesium Hydroxide And Hydrogen Gas Formula Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Molar masses of elements in reactants atomic mass. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. The equation for the reactions of any of these metals would. Magnesium Hydroxide And Hydrogen Gas Formula.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Magnesium Hydroxide And Hydrogen Gas Formula Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. Magnesium + water → magnesium hydroxide + dihydrogen. Be sure to indicate the state (s,l,g or aq) for each substance. The solid magnesium hydroxide is added. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science sauce Magnesium Hydroxide And Hydrogen Gas Formula The equation for the reactions of any of these metals would be: Write a balanced equation for this reaction. Be sure to indicate the state (s,l,g or aq) for each substance. X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. Magnesium hydroxide + hydrogen chloride = magnesium. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.youtube.com
Equation for Magnesium Hydroxide Dissolving in Water Mg(OH)2 + H2O Magnesium Hydroxide And Hydrogen Gas Formula \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. Write a balanced equation for this reaction. Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. The equation for the reactions of any of these metals would be: Be sure to indicate the state (s,l,g or aq) for each substance. Magnesium burns in steam to produce white magnesium. Magnesium Hydroxide And Hydrogen Gas Formula.
From exopiharb.blob.core.windows.net
Magnesium Hydroxide And Formula at Mary Starkes blog Magnesium Hydroxide And Hydrogen Gas Formula Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Write a balanced equation for this reaction. The equation for the reactions of any of these metals would be: Be sure to indicate the state (s,l,g or aq) for each substance. Magnesium + water → magnesium hydroxide. Magnesium Hydroxide And Hydrogen Gas Formula.
From animalia-life.club
Magnesium Hydroxide Formula Magnesium Hydroxide And Hydrogen Gas Formula X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. The equation for the reactions of any of these metals would be: M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. Write a balanced equation for this reaction. Magnesium hydroxide + hydrogen chloride = magnesium chloride. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.numerade.com
Magnesium powder reacts with steam to form magnesium hydroxide and Magnesium Hydroxide And Hydrogen Gas Formula Magnesium + water → magnesium hydroxide + dihydrogen. Write a balanced equation for this reaction. Mg(oh)2 + hcl = mgcl2 + h2o is a double displacement. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. X (s) + 2h 2. Magnesium Hydroxide And Hydrogen Gas Formula.
From ar.inspiredpencil.com
Magnesium Hydroxide Structure Magnesium Hydroxide And Hydrogen Gas Formula M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. Write a balanced equation for this reaction. Magnesium + water → magnesium hydroxide + dihydrogen. Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. Magnesium. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.chegg.com
Solved 2. Write the balanced chemical equation for A. the Magnesium Hydroxide And Hydrogen Gas Formula Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. Mg(oh)2 + hcl = mgcl2 + h2o is a double displacement. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. Magnesium + water → magnesium hydroxide + dihydrogen. M g(s) + 2h 2o(l) → m g(oh)2(aq). Magnesium Hydroxide And Hydrogen Gas Formula.
From www.slideshare.net
Chemical Reactions Magnesium Hydroxide And Hydrogen Gas Formula The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Mg(oh)2 + hcl = mgcl2 + h2o is a double displacement. Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. X (s) + 2h 2 o (l) x (oh) 2. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.toppr.com
Write the chemical formula of magnesium hydroxide. Magnesium Hydroxide And Hydrogen Gas Formula The equation for the reactions of any of these metals would be: X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Magnesium hydroxide + hydrogen chloride = magnesium chloride. Magnesium Hydroxide And Hydrogen Gas Formula.
From byjus.com
In the given reaction, Mg + 2HCl Gives MgCl2 + H2 Calculate the volume Magnesium Hydroxide And Hydrogen Gas Formula The equation for the reactions of any of these metals would be: \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. Be sure to indicate the state (s,l,g or aq) for each substance. Write a balanced equation for this reaction. M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. Magnesium hydroxide + hydrogen chloride = magnesium. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.slideserve.com
PPT Chapter Seven Chemical Reactions PowerPoint Presentation, free Magnesium Hydroxide And Hydrogen Gas Formula Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. Be sure to indicate the state (s,l,g or aq) for each substance. Mg(oh)2 + hcl = mgcl2 + h2o is a double displacement. Molar masses of elements in reactants atomic mass. The solid magnesium hydroxide is added to a. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.chegg.com
Solved 1. Magnesium reacts with water to form magnesium Magnesium Hydroxide And Hydrogen Gas Formula Be sure to indicate the state (s,l,g or aq) for each substance. Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. The equation for the reactions of any of these metals would be: Magnesium burns in steam. Magnesium Hydroxide And Hydrogen Gas Formula.
From brainly.in
magnesium react with stream to give magnesium hydroxide and hydrogen Magnesium Hydroxide And Hydrogen Gas Formula The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Mg(oh)2 + hcl = mgcl2 + h2o is. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.youtube.com
Reaction between Magnesium and Water (Mg + H2O) YouTube Magnesium Hydroxide And Hydrogen Gas Formula Molar masses of elements in reactants atomic mass. The equation for the reactions of any of these metals would be: The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. Magnesium. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.slideserve.com
PPT Properties of Matter PowerPoint Presentation ID2861758 Magnesium Hydroxide And Hydrogen Gas Formula Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. The equation for the reactions of any of these metals would be: Mg(oh)2 + hcl = mgcl2 + h2o is a double displacement. Be sure to indicate the state (s,l,g or aq) for each substance. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.numerade.com
SOLVEDThe reaction between magnesium metal and water (l) produces Magnesium Hydroxide And Hydrogen Gas Formula Write a balanced equation for this reaction. Magnesium reacts with water to form magnesium hydroxide and hydrogen gas. Mg(oh)2 + hcl = mgcl2 + h2o is a double displacement. Magnesium + water → magnesium hydroxide + dihydrogen. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. The equation for the reactions. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.slideserve.com
PPT Mg(OH) 2 + 2HCl → 2H 2 O + MgCl 2 PowerPoint Presentation, free Magnesium Hydroxide And Hydrogen Gas Formula Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. Be sure to indicate the state (s,l,g or aq) for each substance. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. X (s) + 2h 2 o (l) x (oh) 2 (aq or s) + h 2 (g) the hydroxides aren't very soluble,. Magnesium + water → magnesium. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.chegg.com
Solved 2. In a reaction between magnesium hydroxide and Magnesium Hydroxide And Hydrogen Gas Formula Be sure to indicate the state (s,l,g or aq) for each substance. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. Write a balanced equation for this reaction. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas.. Magnesium Hydroxide And Hydrogen Gas Formula.
From www.numerade.com
SOLVED Observations 1, Sodium metal water Word equation sodium metal Magnesium Hydroxide And Hydrogen Gas Formula Magnesium hydroxide + hydrogen chloride = magnesium chloride + water. Write a balanced equation for this reaction. Magnesium + water → magnesium hydroxide + dihydrogen. M g(s) + 2h 2o(l) → m g(oh)2(aq) +h 2(g) ↑⏐. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Magnesium. Magnesium Hydroxide And Hydrogen Gas Formula.