Regulatory Labeling Requirements . Any hearing aid that does not satisfy the requirements of § 800.30. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. This section specifies the labeling requirements for prescription hearing aids.
from regulationlatest.blogspot.com
The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. Any hearing aid that does not satisfy the requirements of § 800.30. This section specifies the labeling requirements for prescription hearing aids. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other.
Fda Regulations For Food Manufacturing
Regulatory Labeling Requirements This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. Any hearing aid that does not satisfy the requirements of § 800.30. The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. This section specifies the labeling requirements for prescription hearing aids. This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other.
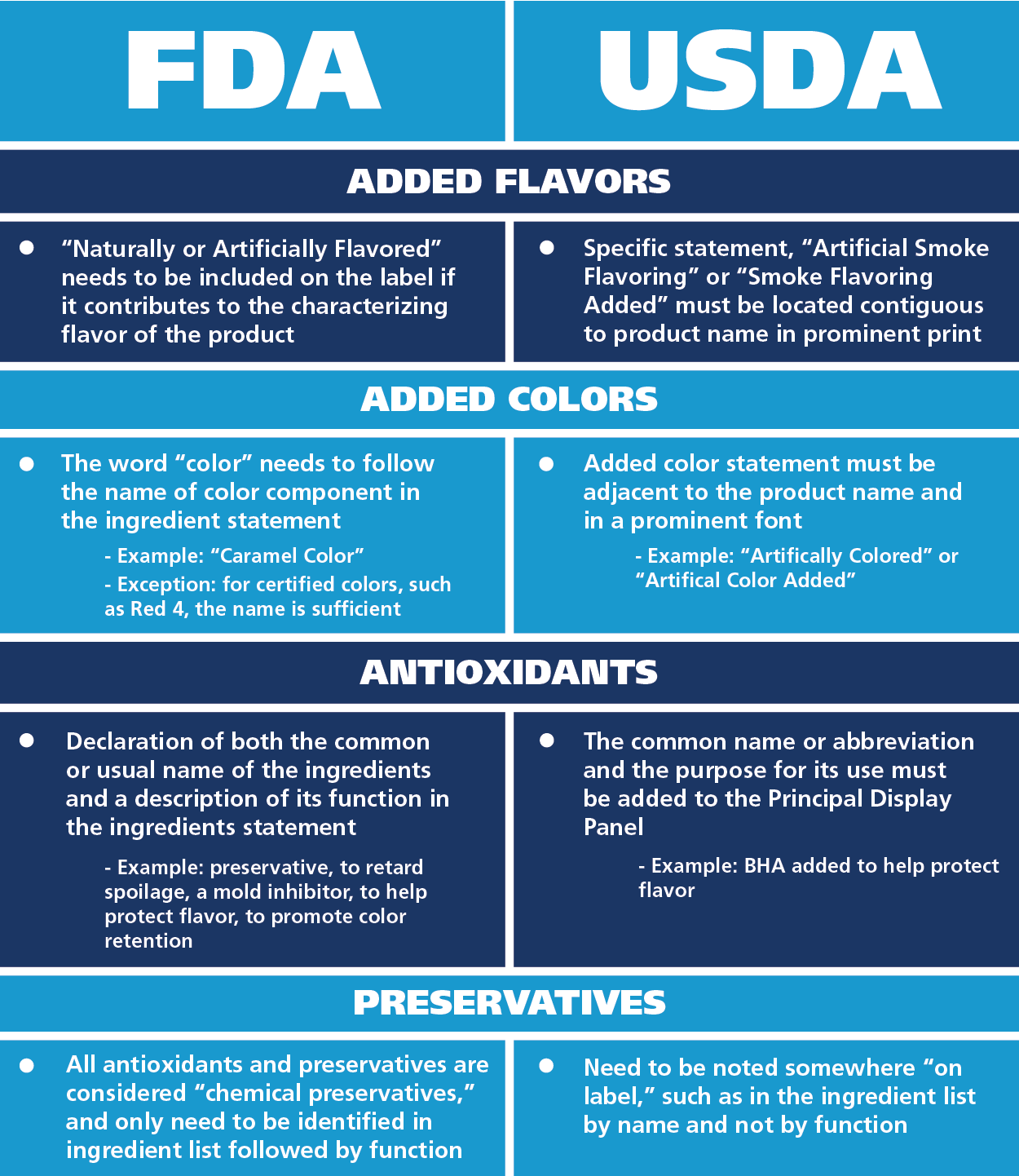
From www.vrogue.co
Modifications To Fda Food Labeling Requirements Tempo vrogue.co Regulatory Labeling Requirements The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. Any hearing aid that does not satisfy the requirements of § 800.30. This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. This section specifies the. Regulatory Labeling Requirements.
From www.dennybros.com
How to stay compliant when labelling cosmetics Regulatory Labeling Requirements This section specifies the labeling requirements for prescription hearing aids. This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. These fda food labeling web pages. Regulatory Labeling Requirements.
From www.slideserve.com
PPT Legal Requirements of Labelling as imposed by the Food Labelling Regulatory Labeling Requirements This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. This section specifies the labeling requirements for prescription hearing aids. The. Regulatory Labeling Requirements.
From www.hazmatuniversity.com
IATA Dangerous Goods Training Hazmat University Regulatory Labeling Requirements (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. Any hearing aid that does not satisfy the requirements of § 800.30. This section specifies the labeling requirements for prescription hearing aids. These fda food labeling web pages address the labeling requirements for. Regulatory Labeling Requirements.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Regulatory Labeling Requirements These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. Any hearing aid that does not satisfy the requirements of § 800.30. This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. (2) when such. Regulatory Labeling Requirements.
From recycleme.eco
RecycleMe Labelling requirements for packaging in Europe Regulatory Labeling Requirements The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. This section specifies the labeling requirements for prescription hearing aids. This guide serves as an. Regulatory Labeling Requirements.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Regulatory Labeling Requirements (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. This section specifies the labeling requirements for prescription hearing aids. Any hearing aid that does not satisfy the requirements of § 800.30. These fda food labeling web pages address the labeling requirements for. Regulatory Labeling Requirements.
From www.futures-supplies.co.uk
Regulations News Flash Regulatory Labeling Requirements These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. This section specifies the labeling requirements for prescription hearing aids. Any hearing aid that does not satisfy the requirements of § 800.30. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting. Regulatory Labeling Requirements.
From labelbasic.com
CBD Labeling Regulations For Packaging Products Regulatory Labeling Requirements These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. This section specifies the labeling requirements for prescription hearing aids. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not.. Regulatory Labeling Requirements.
From www.nist.gov
Packaging and Labeling Timeline Diagram Regulatory Labeling Requirements These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. This section specifies the labeling requirements for prescription hearing aids. Any hearing aid that does not satisfy the requirements of § 800.30. This guide serves as an introduction to us product labeling and marking requirements for clothing,. Regulatory Labeling Requirements.
From emmainternational.com
The Importance of FDA Packaging & Labeling Regulations Regulatory Labeling Requirements The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. This section specifies the labeling requirements for prescription hearing aids. Any hearing aid that does not satisfy the requirements of § 800.30. This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics,. Regulatory Labeling Requirements.
From regulationlatest.blogspot.com
Fda Regulations For Food Manufacturing Regulatory Labeling Requirements (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. Any hearing aid that does not satisfy the requirements of § 800.30. The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and. Regulatory Labeling Requirements.
From ofpack.com.au
Food Labeling Rules And Regulations In Australia Regulatory Labeling Requirements These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. Any hearing aid that does not satisfy the requirements of § 800.30. This section specifies. Regulatory Labeling Requirements.
From www.freyrsolutions.com
Labeling Requirements for Chemical Products in China Freyr Global Regulatory Labeling Requirements This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. This section specifies the labeling requirements for prescription hearing aids. These. Regulatory Labeling Requirements.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Regulatory Labeling Requirements These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. Any hearing aid that does not satisfy the requirements of. Regulatory Labeling Requirements.
From www.mgscientific.com
MG Scientific GHS Compliance Standards Regulatory Labeling Requirements This section specifies the labeling requirements for prescription hearing aids. These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not.. Regulatory Labeling Requirements.
From www.general-data.com
GHS Compliant Labels and GHS Labeling Systems General Data Regulatory Labeling Requirements (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. This section specifies the labeling requirements for prescription hearing aids. Any hearing aid that does not satisfy the requirements of § 800.30. This guide serves as an introduction to us product labeling and. Regulatory Labeling Requirements.
From ar.inspiredpencil.com
Fda Labeling Regulations Regulatory Labeling Requirements This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of. Regulatory Labeling Requirements.
From medicaldeviceacademy.com
How to Audit Your Labeling Process for 21 CFR 820 Compliance Regulatory Labeling Requirements This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. This section specifies the labeling requirements for prescription hearing aids. (2) when such cosmetics are. Regulatory Labeling Requirements.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Regulatory Labeling Requirements This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. The fair packaging and labeling act (fpla or act), enacted in. Regulatory Labeling Requirements.
From foodindustryexecutive.com
FDA Final Guidance Clarifies New Nutrition Label Requirements Food Regulatory Labeling Requirements The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. This section specifies the labeling requirements for prescription hearing aids. This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. (2) when such cosmetics are sold. Regulatory Labeling Requirements.
From www.vrogue.co
Food Labeling 101 Fda Regulations Guide 2022 Artwork vrogue.co Regulatory Labeling Requirements This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal. Regulatory Labeling Requirements.
From www.foodlogistics.com
How to Respond Quickly to Customer and Regulatory Labeling Requirements Regulatory Labeling Requirements The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. This section specifies the labeling requirements for prescription hearing aids. This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. (2) when such cosmetics are sold. Regulatory Labeling Requirements.
From juleteagyd.blogspot.com
Ghs Secondary Container Label Requirements Juleteagyd Regulatory Labeling Requirements This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal. Regulatory Labeling Requirements.
From www.pinterest.com
Summary of Cosmetics Labeling Requirements Labels, Regulators, Summary Regulatory Labeling Requirements This section specifies the labeling requirements for prescription hearing aids. The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. Any hearing aid that does not satisfy the requirements of § 800.30. These fda food labeling web pages address the labeling requirements for foods under the federal food,. Regulatory Labeling Requirements.
From www.tailoredlabel.com
ANSI Label Standards & Warning Label Requirements TLP Regulatory Labeling Requirements These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting. Regulatory Labeling Requirements.
From www.fdabasics.com
USA Food Labeling Regulations FDABasics Regulatory Labeling Requirements Any hearing aid that does not satisfy the requirements of § 800.30. The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. This section specifies. Regulatory Labeling Requirements.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Regulatory Labeling Requirements Any hearing aid that does not satisfy the requirements of § 800.30. This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is. Regulatory Labeling Requirements.
From www.avery.com
4 Quick Tips to Help You Ace OSHA Secondary Container Labeling Regulatory Labeling Requirements These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug. Any hearing aid that does not satisfy the requirements of § 800.30. This section specifies. Regulatory Labeling Requirements.
From thecompliancepeople.co.uk
Requirements to comply with Classification Labelling Packaging Regulatory Labeling Requirements These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. Any hearing aid that does not satisfy the requirements of § 800.30. This section specifies the labeling requirements for prescription hearing aids. This guide serves as an introduction to us product labeling and marking requirements for clothing,. Regulatory Labeling Requirements.
From packaginghub.com
FDA Packaging and Labeling Requirements Guide Packaging Hub Regulatory Labeling Requirements (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. The fair packaging and labeling act (fpla or act), enacted in. Regulatory Labeling Requirements.
From ar.inspiredpencil.com
Fda Labeling Requirements For Food Regulatory Labeling Requirements (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. This section specifies the labeling requirements for prescription hearing aids. Any hearing aid that does not satisfy the requirements of § 800.30. These fda food labeling web pages address the labeling requirements for. Regulatory Labeling Requirements.
From ar.inspiredpencil.com
Fda Labeling Regulations Regulatory Labeling Requirements This section specifies the labeling requirements for prescription hearing aids. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments.. Regulatory Labeling Requirements.
From www.labelmaster.com
Globally Harmonized System GHS from Labelmaster Regulatory Labeling Requirements This section specifies the labeling requirements for prescription hearing aids. (2) when such cosmetics are sold at retail as part of a cosmetic package consisting of an inner and outer container and the inner container is not. This guide serves as an introduction to us product labeling and marking requirements for clothing, electronics, children’s products, furniture, and many other. These. Regulatory Labeling Requirements.
From regask.com
Regulatory Labeling RegASK Regulatory Labeling Requirements These fda food labeling web pages address the labeling requirements for foods under the federal food, drug, and cosmetic act and its amendments. This section specifies the labeling requirements for prescription hearing aids. Any hearing aid that does not satisfy the requirements of § 800.30. The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal. Regulatory Labeling Requirements.