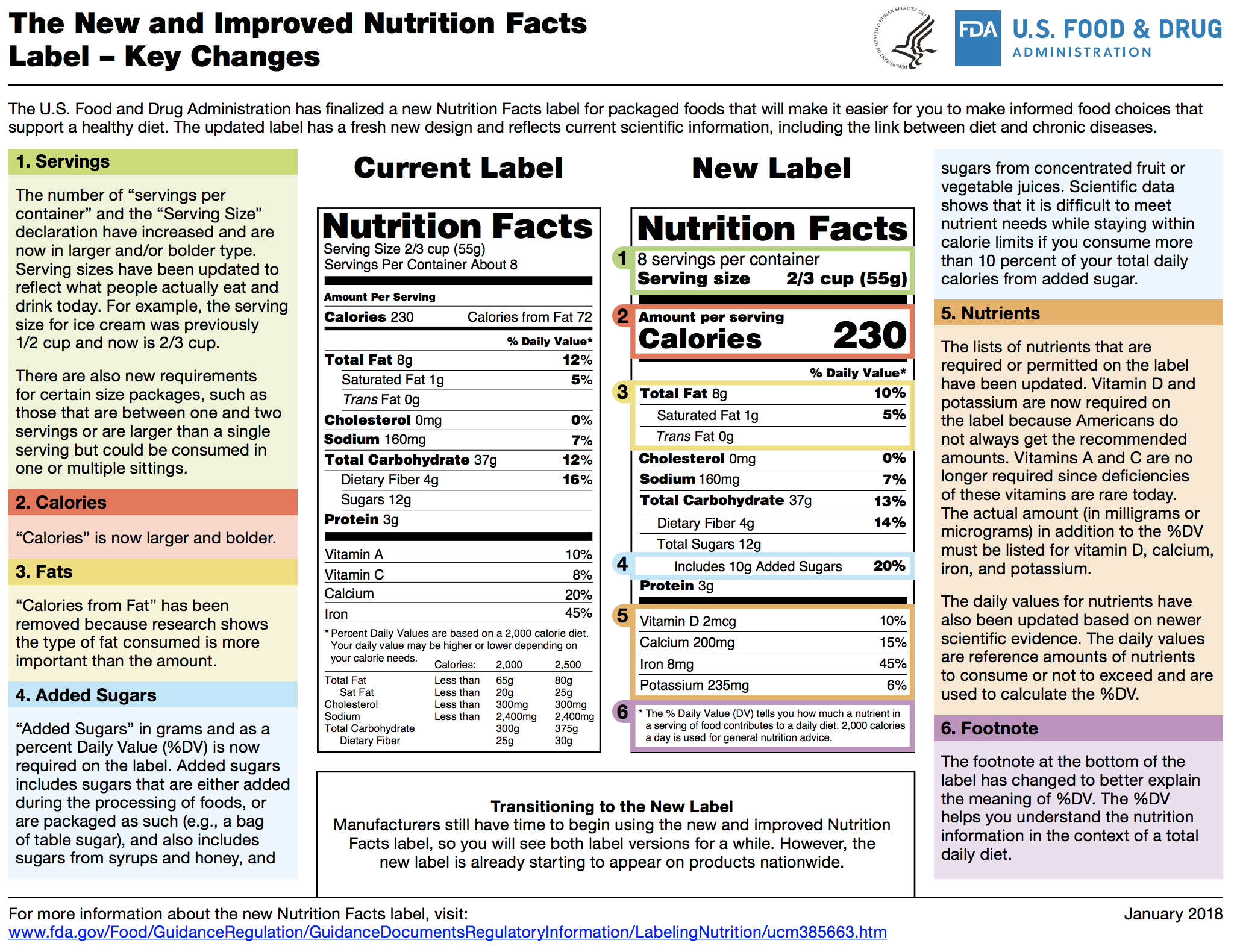
Fda Dietary Supplements Guidance . guidance and regulatory information on food and dietary supplements; Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. draft guidance for industry: a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. Guidance documents represent fda's current. 278 rows the documents listed below are guidance for the food industry. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an.
from foodindustryexecutive.com
guidance and regulatory information on food and dietary supplements; a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. draft guidance for industry: the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. Guidance documents represent fda's current. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an. 278 rows the documents listed below are guidance for the food industry.
FDA Final Guidance Clarifies New Nutrition Label Requirements Food
Fda Dietary Supplements Guidance the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. draft guidance for industry: guidance and regulatory information on food and dietary supplements; (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an. the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. 278 rows the documents listed below are guidance for the food industry. the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. Guidance documents represent fda's current.
From synthys-medical.com
FDA Dietary Supplements SYNTHYS MEDICAL Fda Dietary Supplements Guidance (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an. 278 rows the documents listed below are guidance for the food industry. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. Policy regarding certain new dietary. Fda Dietary Supplements Guidance.
From www.scribd.com
Dietary Supplement GMP.2012 Food And Drug Administration Hazard Fda Dietary Supplements Guidance draft guidance for industry: the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. Guidance documents represent fda's current. 278 rows the documents listed below are guidance for the food industry. guidance and regulatory information on food and dietary supplements; the federal food, drug, and cosmetic act. Fda Dietary Supplements Guidance.
From theherbalacademy.com
What FDA Dietary Supplement Regulations Mean For Herbalists Fda Dietary Supplements Guidance the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. draft guidance for industry: Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. (a) the label of. Fda Dietary Supplements Guidance.
From www.healthdigest.com
The FDA Has A New Guide For Dietary Supplements. Here's How To Use It Fda Dietary Supplements Guidance 278 rows the documents listed below are guidance for the food industry. draft guidance for industry: Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. the federal food, drug, and cosmetic act (fdca) defines certain. Fda Dietary Supplements Guidance.
From affiliatenutra.com
Guide to FDA and Nutraceutical Supplement Label Requirements Fda Dietary Supplements Guidance Guidance documents represent fda's current. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an. the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. the federal food, drug, and cosmetic act (fdca) defines certain foods. Fda Dietary Supplements Guidance.
From gossiphealth.com
How to Use New FDA Guidance for Dietary Supplements Gossip Health Fda Dietary Supplements Guidance Guidance documents represent fda's current. draft guidance for industry: 278 rows the documents listed below are guidance for the food industry. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. guidance and regulatory information on food and dietary supplements; Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety. Fda Dietary Supplements Guidance.
From cegmcylp.blob.core.windows.net
Fda Food Code Supplement at Ignacio McCord blog Fda Dietary Supplements Guidance a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. draft guidance for industry: the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. guidance and regulatory. Fda Dietary Supplements Guidance.
From rasladeloris.pages.dev
Fda Food Labeling Guide 2025 Pauli Bethanne Fda Dietary Supplements Guidance the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. draft guidance for industry: Guidance documents represent fda's current. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an. Policy regarding certain new dietary ingredients and dietary supplements subject to. Fda Dietary Supplements Guidance.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Fda Dietary Supplements Guidance the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. 278 rows the documents listed below are guidance for the food industry.. Fda Dietary Supplements Guidance.
From www.fdalisting.com
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements Fda Dietary Supplements Guidance (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an. the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. Guidance documents. Fda Dietary Supplements Guidance.
From foodindustryexecutive.com
FDA Final Guidance Clarifies New Nutrition Label Requirements Food Fda Dietary Supplements Guidance Guidance documents represent fda's current. the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. guidance and regulatory information on food and. Fda Dietary Supplements Guidance.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Fda Dietary Supplements Guidance (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an. 278 rows the documents listed below are guidance for the food industry. a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. Guidance documents represent fda's. Fda Dietary Supplements Guidance.
From ihrmagazine.com
New FDA Draft Guidance Aims to Increase Safety Information About Fda Dietary Supplements Guidance guidance and regulatory information on food and dietary supplements; Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. 278 rows the documents listed below are guidance for the food industry. the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. a new draft guidance from the fda indicates. Fda Dietary Supplements Guidance.
From www.slideshare.net
FDA's Guidance On Beverages Vs Dietary Supplements Fda Dietary Supplements Guidance the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an. guidance and regulatory information on food and dietary supplements; Food and drug administration (fda), which regulates. Fda Dietary Supplements Guidance.
From www.slideshare.net
FDA's Guidance On Beverages Vs Dietary Supplements Fda Dietary Supplements Guidance guidance and regulatory information on food and dietary supplements; draft guidance for industry: the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. Policy regarding certain new dietary ingredients and dietary supplements subject to. Fda Dietary Supplements Guidance.
From www.slideshare.net
FDA's Guidance On Beverages Vs Dietary Supplements Fda Dietary Supplements Guidance a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. Guidance documents represent fda's current. Policy regarding certain. Fda Dietary Supplements Guidance.
From www.rimonlaw.com
Supplement Industry Stays On FDA’s Radar with New Draft Guidance for Fda Dietary Supplements Guidance the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. Guidance documents represent fda's current. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. draft guidance for industry: a new draft. Fda Dietary Supplements Guidance.
From www.slideserve.com
PPT REGULATION OF DIETARY SUPPLEMENTS PowerPoint Presentation, free Fda Dietary Supplements Guidance a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. guidance and regulatory information on food and dietary supplements;. Fda Dietary Supplements Guidance.
From www.slideserve.com
PPT FDA Dietary Supplement Update PowerPoint Presentation, free Fda Dietary Supplements Guidance Guidance documents represent fda's current. guidance and regulatory information on food and dietary supplements; a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. 278 rows the documents listed below are guidance for the food industry. the federal food, drug, and cosmetic act (fdca) defines certain foods as. Fda Dietary Supplements Guidance.
From nutritionpics.blogspot.com
Are Nutritional Supplements Regulated By The Fda Nutrition Pics Fda Dietary Supplements Guidance 278 rows the documents listed below are guidance for the food industry. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. draft guidance for industry: a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. the food and drug administration (fda or we) is. Fda Dietary Supplements Guidance.
From www.paragonlabsusa.com
Guide to FDA Supplement Label Requirements Paragon Laboratories Fda Dietary Supplements Guidance 278 rows the documents listed below are guidance for the food industry. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. draft guidance for industry: guidance and regulatory information. Fda Dietary Supplements Guidance.
From www.slideshare.net
FDA's Guidance On Beverages Vs Dietary Supplements Fda Dietary Supplements Guidance a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. guidance and regulatory information on food and dietary supplements; Guidance documents represent fda's current. draft guidance for industry: Food and. Fda Dietary Supplements Guidance.
From studylib.net
FDA Product Data Sheet Dietary Supplements Fda Dietary Supplements Guidance the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. guidance and regulatory information on food and dietary supplements; a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. 278 rows the documents listed below are guidance for the food industry. Policy regarding certain. Fda Dietary Supplements Guidance.
From www.healthline.com
How to Use New FDA Guidance for Dietary Supplements Fda Dietary Supplements Guidance 278 rows the documents listed below are guidance for the food industry. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. draft guidance for industry: the federal food, drug, and cosmetic act (fdca) defines certain. Fda Dietary Supplements Guidance.
From www.detrester.com
Dietary Supplement Label Template Fda Dietary Supplements Guidance the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an. 278 rows. Fda Dietary Supplements Guidance.
From vipcolor.com
The eight things that you must know about food supplement labels. And Fda Dietary Supplements Guidance 278 rows the documents listed below are guidance for the food industry. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. Policy regarding certain new dietary. Fda Dietary Supplements Guidance.
From www.slideshare.net
FDA Guidance On Liquid Dietary Supplements Vs. Beverages Commentary J… Fda Dietary Supplements Guidance a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. guidance and regulatory information on food and dietary supplements; Guidance documents represent fda's current. Food and drug administration (fda), which regulates. Fda Dietary Supplements Guidance.
From www.slideserve.com
PPT FDA Dietary Supplement Update PowerPoint Presentation, free Fda Dietary Supplements Guidance the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any. Fda Dietary Supplements Guidance.
From cohenhealthcarelaw.com
FDA Dietary Supplement Labeling Requirements Comply or Die Cohen Fda Dietary Supplements Guidance (a) the label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an. the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. guidance and regulatory. Fda Dietary Supplements Guidance.
From www.vitamixlabs.com
Labeling a Dietary Supplement Fda Dietary Supplements Guidance Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. guidance and regulatory information on food and dietary supplements; Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. a new draft guidance from the fda indicates that the agency may gather more safety information on dietary.. Fda Dietary Supplements Guidance.
From cekhhzie.blob.core.windows.net
Dietary Supplement Defined By Fda at Patrick Hoffman blog Fda Dietary Supplements Guidance the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. Guidance documents represent fda's current. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. Food and drug administration (fda), which regulates dietary. Fda Dietary Supplements Guidance.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Fda Dietary Supplements Guidance Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. draft guidance for industry: Guidance documents represent fda's current. the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. the federal food, drug, and cosmetic act (fdca) defines certain foods as. Fda Dietary Supplements Guidance.
From easconsultinggroup.com
Dietary Supplement Labeling Handbook A Guide for 21 CFR 101 Fda Dietary Supplements Guidance Guidance documents represent fda's current. the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. 278 rows the documents listed below are. Fda Dietary Supplements Guidance.
From www.lachmanconsultants.com
FDA Adds New and Revised Bioequivalence Guidances Lachman Consultants Fda Dietary Supplements Guidance Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. a new draft guidance from the fda indicates that the agency may gather more safety information on dietary. Food and drug administration (fda), which regulates dietary supplements, requires that companies submit safety data about any new. the federal food, drug, and cosmetic act (fdca) defines. Fda Dietary Supplements Guidance.
From nutritionpics.blogspot.com
Are Nutritional Supplements Regulated By The Fda Nutrition Pics Fda Dietary Supplements Guidance Policy regarding certain new dietary ingredients and dietary supplements subject to the requirement. the food and drug administration (fda or we) is announcing the availability of a final guidance for industry. draft guidance for industry: the federal food, drug, and cosmetic act (fdca) defines certain foods as dietary supplements. Food and drug administration (fda), which regulates dietary. Fda Dietary Supplements Guidance.