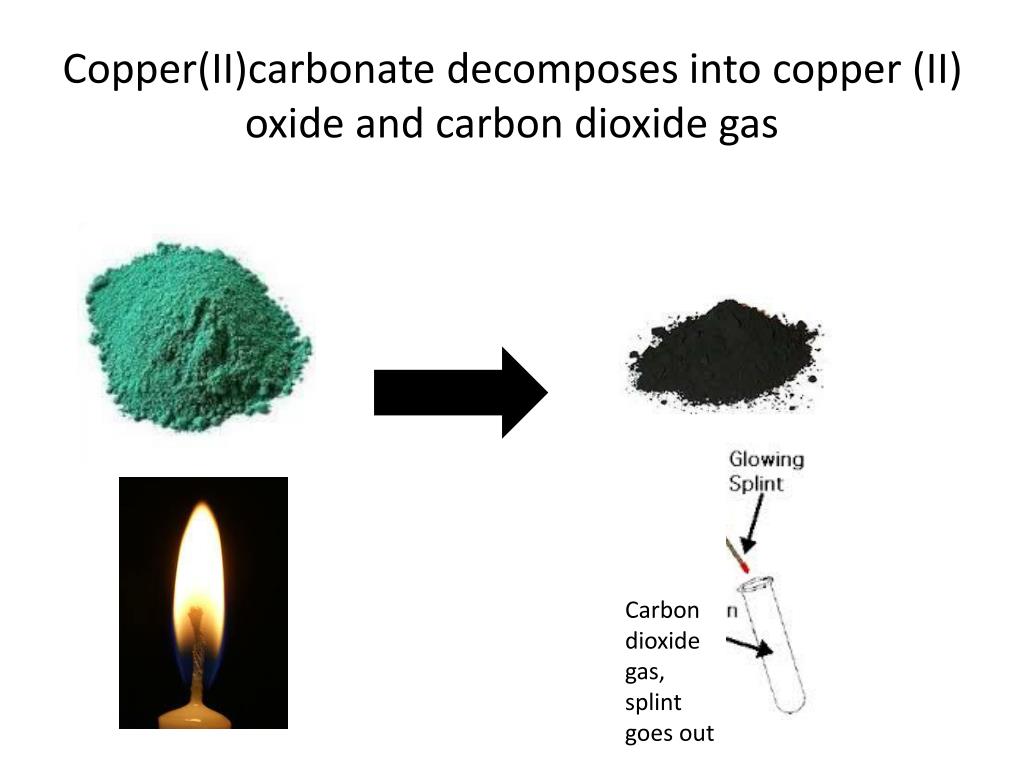
Copper Hydroxide Decomposes . Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. 3d, of c /4=1.3 å), arises to put these copper atoms at equal. In addition, a second shift of half the copper atoms (shift 2 on fig. This material does not contribute to co2 bubbling in glazes. The reaction is represented by the equation cu(oh)₂ → cuo +. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated.
from www.slideserve.com
Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. The reaction is represented by the equation cu(oh)₂ → cuo +. 3d, of c /4=1.3 å), arises to put these copper atoms at equal. In addition, a second shift of half the copper atoms (shift 2 on fig. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated. This material does not contribute to co2 bubbling in glazes.
PPT Types of Reactions Lab PowerPoint Presentation, free download
Copper Hydroxide Decomposes So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. The reaction is represented by the equation cu(oh)₂ → cuo +. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. This material does not contribute to co2 bubbling in glazes. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. 3d, of c /4=1.3 å), arises to put these copper atoms at equal. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. In addition, a second shift of half the copper atoms (shift 2 on fig. So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated.
From www.youtube.com
Copper Hydroxide YouTube Copper Hydroxide Decomposes Copper hydroxide has a fairly complex decomposition as it is heated to melting point. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. 3d, of c /4=1.3 å), arises to put these copper atoms at equal. In addition, a second shift of half the copper atoms (shift 2 on fig. So. Copper Hydroxide Decomposes.
From ar.inspiredpencil.com
Copper Hydroxide Solution Copper Hydroxide Decomposes So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. This material does not contribute to co2 bubbling in glazes. The reaction is represented by the equation cu(oh)₂ →. Copper Hydroxide Decomposes.
From www.numerade.com
SOLVED Balance the equations or write the balanced chemical for the Copper Hydroxide Decomposes 3d, of c /4=1.3 å), arises to put these copper atoms at equal. In addition, a second shift of half the copper atoms (shift 2 on fig. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. This material does not contribute to co2 bubbling in glazes. Depending on how it was made, a sample of copper (ii). Copper Hydroxide Decomposes.
From www.youtube.com
of CopperII Carbonate Hydroxide Hydrate Lab Video YouTube Copper Hydroxide Decomposes Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated. The production of copper (ii). Copper Hydroxide Decomposes.
From www.linkedin.com
What Does Copper Hydroxide Fungicide Do? Copper Hydroxide Decomposes The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. The reaction is represented by the equation cu(oh)₂ → cuo +. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. In addition, a second shift. Copper Hydroxide Decomposes.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide Decomposes Copper hydroxide has a fairly complex decomposition as it is heated to melting point. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. This material does not contribute. Copper Hydroxide Decomposes.
From www.indiamart.com
Copper Hydroxide at Rs 525/kg Copper Hydroxide in Hyderabad ID Copper Hydroxide Decomposes In addition, a second shift of half the copper atoms (shift 2 on fig. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air,. Copper Hydroxide Decomposes.
From www.wonano.com
Copper Hydroxide Nanorods, NanoCupric Powder, Cupric Hydroxide Nanopowder Copper Hydroxide Decomposes 3d, of c /4=1.3 å), arises to put these copper atoms at equal. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. In addition, a second shift of half the copper. Copper Hydroxide Decomposes.
From www.shutterstock.com
Copper Hydroxide Properties Chemical Compound Structure Stock Vector Copper Hydroxide Decomposes Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. The production of copper (ii) hydroxide typically. Copper Hydroxide Decomposes.
From www.onlinemathlearning.com
of Metal Compounds (solutions, examples, activities Copper Hydroxide Decomposes So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated. This material does not contribute to co2 bubbling in glazes. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. The reaction is represented by the equation cu(oh)₂ →. Copper Hydroxide Decomposes.
From fphoto.photoshelter.com
science chemical reaction equilibrium cupric carbonate Fundamental Copper Hydroxide Decomposes 3d, of c /4=1.3 å), arises to put these copper atoms at equal. This material does not contribute to co2 bubbling in glazes. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. In addition, a second shift of half. Copper Hydroxide Decomposes.
From www.youtube.com
Making copper hydroxide YouTube Copper Hydroxide Decomposes 3d, of c /4=1.3 å), arises to put these copper atoms at equal. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. In addition, a second shift of half the copper atoms (shift 2 on fig. The reaction is represented by the equation. Copper Hydroxide Decomposes.
From www.youtube.com
Precipitation Reactions Copper Hydroxide Junior YouTube Copper Hydroxide Decomposes The reaction is represented by the equation cu(oh)₂ → cuo +. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. 3d, of c /4=1.3 å), arises to put these copper atoms at equal. So just wondering, copper (ii) hydroxide undergoes. Copper Hydroxide Decomposes.
From loeaiwybj.blob.core.windows.net
Copper Hydroxide Bond at Ernest Boyle blog Copper Hydroxide Decomposes The reaction is represented by the equation cu(oh)₂ → cuo +. In addition, a second shift of half the copper atoms (shift 2 on fig. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. This material does not contribute to. Copper Hydroxide Decomposes.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Copper Hydroxide Decomposes 3d, of c /4=1.3 å), arises to put these copper atoms at equal. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. This material does not contribute to co2 bubbling in glazes. So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated. The production of copper (ii) hydroxide typically occurs through the reaction. Copper Hydroxide Decomposes.
From www.slideserve.com
PPT Types of Reactions Lab PowerPoint Presentation, free download Copper Hydroxide Decomposes Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. This material does not contribute to co2 bubbling in glazes. So just wondering, copper (ii) hydroxide undergoes thermal decomposition. Copper Hydroxide Decomposes.
From fineartamerica.com
Precipitation Of Copper (ii) Hydroxide Photograph by David Taylor Copper Hydroxide Decomposes Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. In addition, a second shift of half the copper atoms (shift 2 on fig. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. Copper (ii) hydroxide. Copper Hydroxide Decomposes.
From www.scribd.com
Synthesis of Copper Hydroxide3 PDF Stoichiometry Unit Processes Copper Hydroxide Decomposes The reaction is represented by the equation cu(oh)₂ → cuo +. This material does not contribute to co2 bubbling in glazes. In addition, a second shift of half the copper atoms (shift 2 on fig. So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated. The production of copper (ii) hydroxide typically occurs through the reaction of copper. Copper Hydroxide Decomposes.
From www.youtube.com
DIY Making Copper Hydroxide via Electrolysis YouTube Copper Hydroxide Decomposes Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. 3d, of c /4=1.3 å),. Copper Hydroxide Decomposes.
From www.savemyexams.co.uk
Carbon Dioxide from Thermal (2.3.4) Edexcel IGCSE Copper Hydroxide Decomposes Copper hydroxide has a fairly complex decomposition as it is heated to melting point. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. This material does not contribute to co2 bubbling in glazes. 3d, of c /4=1.3 å), arises. Copper Hydroxide Decomposes.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Copper Hydroxide Decomposes So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated. This material does not contribute to co2 bubbling in glazes. In addition, a second shift of half the copper atoms (shift 2 on fig. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii). Copper Hydroxide Decomposes.
From loeaiwybj.blob.core.windows.net
Copper Hydroxide Bond at Ernest Boyle blog Copper Hydroxide Decomposes Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. In addition, a second shift of half the copper atoms (shift 2 on fig. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. This material does not contribute to co2 bubbling in glazes. The production of copper (ii) hydroxide typically occurs through the. Copper Hydroxide Decomposes.
From www.sciencephoto.com
Copper Hydroxide Precipitate Stock Image C027/9450 Science Photo Copper Hydroxide Decomposes Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. This material does not contribute to co2 bubbling in glazes. So just wondering, copper (ii) hydroxide undergoes thermal decomposition. Copper Hydroxide Decomposes.
From www.youtube.com
Copper Hydroxide Synthesis YouTube Copper Hydroxide Decomposes Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. In addition, a second shift of half the copper atoms (shift 2 on fig. This material does not contribute to co2 bubbling in glazes. The reaction is represented by the equation cu(oh)₂ → cuo +. Depending on how it was made, a sample of copper (ii) hydroxide may. Copper Hydroxide Decomposes.
From dir.indiamart.com
Copper Hydroxide at Best Price in India Copper Hydroxide Decomposes The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated. In addition, a second shift of half the copper atoms (shift 2 on fig. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. The reaction is represented. Copper Hydroxide Decomposes.
From es.made-in-china.com
Hidróxido de cobre fungicida WP, del 5077WP China Hidróxido de Copper Hydroxide Decomposes The reaction is represented by the equation cu(oh)₂ → cuo +. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. This material does not contribute to co2 bubbling in glazes. 3d, of c /4=1.3 å), arises to put these copper. Copper Hydroxide Decomposes.
From www.youtube.com
of Copper(II) Carbonate Hydroxide Hydrate in LateNiteLabs Copper Hydroxide Decomposes In addition, a second shift of half the copper atoms (shift 2 on fig. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. 3d, of c /4=1.3 å), arises to put these copper atoms at equal. The reaction is represented by the equation cu(oh)₂ → cuo +. So just wondering, copper (ii) hydroxide undergoes thermal. Copper Hydroxide Decomposes.
From www.sciencephoto.com
Copper Hydroxide Precipitate, 3 of 3 Stock Image C030/8096 Copper Hydroxide Decomposes Copper hydroxide has a fairly complex decomposition as it is heated to melting point. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. 3d, of c /4=1.3 å), arises to put these copper atoms at equal. The reaction is represented. Copper Hydroxide Decomposes.
From www.youtube.com
3. Copper Hydroxide to Copper Oxide (Cu Again Lab) YouTube Copper Hydroxide Decomposes Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. In addition, a second shift of half the copper atoms (shift 2 on fig. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon. Copper Hydroxide Decomposes.
From cepdogcm.blob.core.windows.net
Copper Hydroxide React With Ammonia at Catherine Vasquez blog Copper Hydroxide Decomposes Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. This material does not contribute to co2 bubbling in glazes. In addition, a second shift of half the copper atoms (shift 2 on fig. So just wondering, copper (ii) hydroxide undergoes thermal decomposition when. Copper Hydroxide Decomposes.
From www.youtube.com
Make copper hydroxide with just two compounds YouTube Copper Hydroxide Decomposes Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. In addition, a second shift of half the copper atoms (shift 2 on fig. 3d, of c /4=1.3 å), arises to put these copper atoms at equal. This material does not contribute to co2 bubbling in glazes. Copper hydroxide has a fairly complex decomposition as it is heated. Copper Hydroxide Decomposes.
From www.dreamstime.com
Reaction Copper Carbonate To Copper Oxide and Carbon Copper Hydroxide Decomposes So just wondering, copper (ii) hydroxide undergoes thermal decomposition when it's heated. This material does not contribute to co2 bubbling in glazes. The reaction is represented by the equation cu(oh)₂ → cuo +. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give. Copper Hydroxide Decomposes.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide Decomposes 3d, of c /4=1.3 å), arises to put these copper atoms at equal. Depending on how it was made, a sample of copper (ii) hydroxide may readily absorb carbon dioxide from the air, converting to copper (ii) carbonate, or give off water,. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. The production of copper (ii) hydroxide. Copper Hydroxide Decomposes.
From fphoto.photoshelter.com
science chemical reaction equilibrium cupric carbonate Fundamental Copper Hydroxide Decomposes This material does not contribute to co2 bubbling in glazes. Copper (ii) hydroxide decomposes into copper (ii) oxide and water upon heating. Copper hydroxide has a fairly complex decomposition as it is heated to melting point. 3d, of c /4=1.3 å), arises to put these copper atoms at equal. In addition, a second shift of half the copper atoms (shift. Copper Hydroxide Decomposes.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Copper Hydroxide Decomposes In addition, a second shift of half the copper atoms (shift 2 on fig. The reaction is represented by the equation cu(oh)₂ → cuo +. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. This material does not contribute to co2 bubbling in glazes. 3d, of c /4=1.3 å), arises to. Copper Hydroxide Decomposes.