Does Pressure Increase With Increase In Temperature . When there is an increase in temperature the kinetic energy of gas molecules increases. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. Temperature also illustrates a direct relationship. When the temperature of a system goes up, the pressure also goes up, and vice versa. Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. A graph of pressure vs. When you increase the container volume, you are increasing the degree of freedom of the gas molecules.
from general.chemistrysteps.com
When you increase the container volume, you are increasing the degree of freedom of the gas molecules. A graph of pressure vs. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. When there is an increase in temperature the kinetic energy of gas molecules increases. Temperature also illustrates a direct relationship. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. When the temperature of a system goes up, the pressure also goes up, and vice versa. Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in.
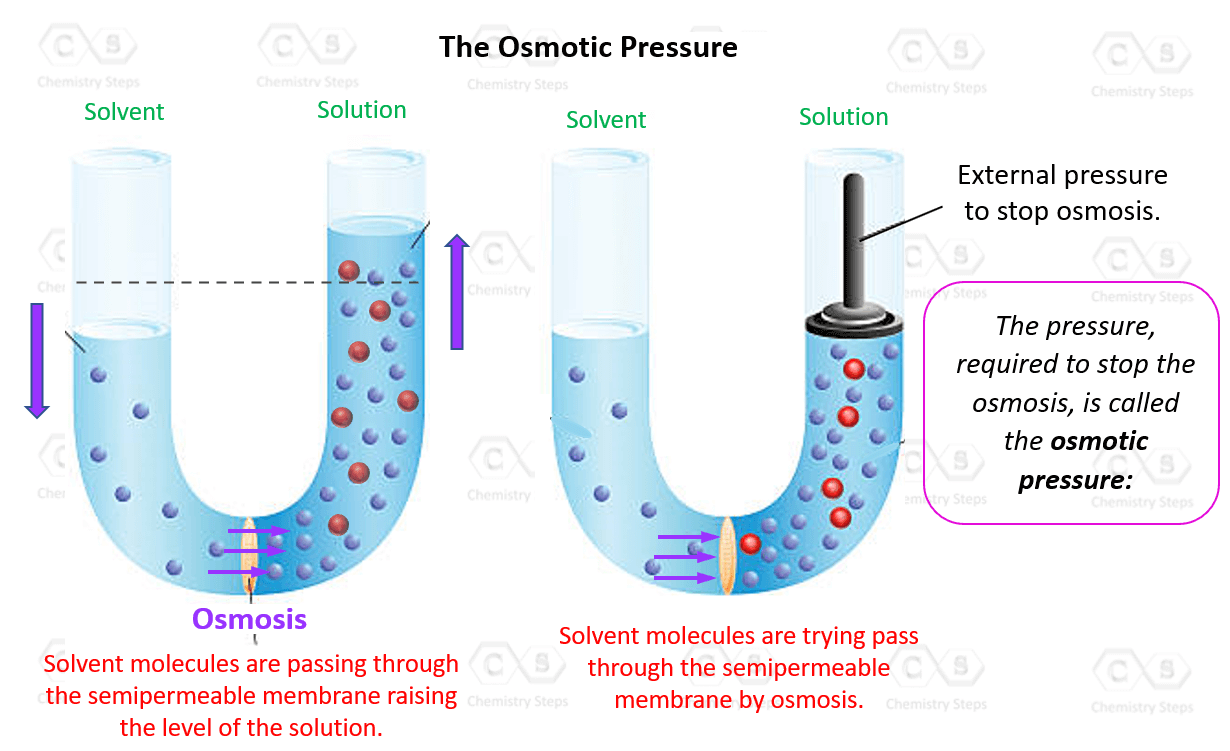
Osmotic Pressure Chemistry Steps
Does Pressure Increase With Increase In Temperature At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. A graph of pressure vs. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. When the temperature of a system goes up, the pressure also goes up, and vice versa. When there is an increase in temperature the kinetic energy of gas molecules increases. Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. When you increase the container volume, you are increasing the degree of freedom of the gas molecules. Temperature also illustrates a direct relationship. The energy added as work during the compression of a gas leads to an increase in pressure and temperature.
From klatordwr.blob.core.windows.net
Does Pressure Rise With Temperature at Eric Thomson blog Does Pressure Increase With Increase In Temperature Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of. When you increase the container volume, you are increasing the degree of freedom of the gas molecules. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. A graph of pressure vs. When. Does Pressure Increase With Increase In Temperature.
From www.britannica.com
perfect gas law chemistry and physics Britannica Does Pressure Increase With Increase In Temperature The energy added as work during the compression of a gas leads to an increase in pressure and temperature. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. Temperature also illustrates a direct relationship. When there is an increase in temperature the kinetic energy of gas molecules increases. A graph of. Does Pressure Increase With Increase In Temperature.
From studyrocket.co.uk
Turning Forces and Pressure GCSE Physics AQA Revision Study Rocket Does Pressure Increase With Increase In Temperature A graph of pressure vs. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. Temperature also illustrates a direct relationship. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. The energy added as work during the compression of a gas leads. Does Pressure Increase With Increase In Temperature.
From slidetodoc.com
The relationship between temperature and volume How Volume Does Pressure Increase With Increase In Temperature Temperature also illustrates a direct relationship. When there is an increase in temperature the kinetic energy of gas molecules increases. A graph of pressure vs. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. The pressure of a given amount of gas is directly proportional to the temperature at a. Does Pressure Increase With Increase In Temperature.
From klaezjnuy.blob.core.windows.net
Pressure Temperature Chart Solid Liquid Gas at Renee Thomas blog Does Pressure Increase With Increase In Temperature When you increase the container volume, you are increasing the degree of freedom of the gas molecules. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. When there is an increase in temperature the kinetic energy of gas molecules increases. Temperature also illustrates a direct relationship. At constant pressure, the volume. Does Pressure Increase With Increase In Temperature.
From www.youtube.com
Effects of Temperature and Pressure on Matter Iken Edu YouTube Does Pressure Increase With Increase In Temperature When there is an increase in temperature the kinetic energy of gas molecules increases. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. When the temperature of a system goes up, the pressure also goes up, and vice versa. When you increase the container volume, you are increasing the degree. Does Pressure Increase With Increase In Temperature.
From stock.adobe.com
Vetor de Charles Law Infographic Diagram Example helium balloon when Does Pressure Increase With Increase In Temperature Temperature also illustrates a direct relationship. When you increase the container volume, you are increasing the degree of freedom of the gas molecules. Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of. A graph of pressure vs. At constant pressure, the volume of a fixed amount of gas is directly proportional. Does Pressure Increase With Increase In Temperature.
From loebantdv.blob.core.windows.net
Why Does Equilibrium Vapor Pressure Increase With Temperature at Thomas Does Pressure Increase With Increase In Temperature Temperature also illustrates a direct relationship. When the temperature of a system goes up, the pressure also goes up, and vice versa. When there is an increase in temperature the kinetic energy of gas molecules increases. When you increase the container volume, you are increasing the degree of freedom of the gas molecules. The energy added as work during the. Does Pressure Increase With Increase In Temperature.
From www.researchgate.net
Variations of the pressure increase, temperature rise, and film Does Pressure Increase With Increase In Temperature Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. When there is an increase in temperature the kinetic energy of gas molecules increases. When the temperature of a system goes up, the. Does Pressure Increase With Increase In Temperature.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Does Pressure Increase With Increase In Temperature Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of. When there is an increase in temperature the kinetic energy of gas molecules increases. A graph of pressure vs. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. The pressure of a. Does Pressure Increase With Increase In Temperature.
From www.slideserve.com
PPT AP Chemistry Unit 2 PowerPoint Presentation, free download ID Does Pressure Increase With Increase In Temperature The energy added as work during the compression of a gas leads to an increase in pressure and temperature. When the temperature of a system goes up, the pressure also goes up, and vice versa. When there is an increase in temperature the kinetic energy of gas molecules increases. Temperature also illustrates a direct relationship. The pressure of a given. Does Pressure Increase With Increase In Temperature.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps Does Pressure Increase With Increase In Temperature The pressure of a given amount of gas is directly proportional to the temperature at a given volume. When there is an increase in temperature the kinetic energy of gas molecules increases. When you increase the container volume, you are increasing the degree of freedom of the gas molecules. Pressure increases with temperature in a closed system with constant volume. Does Pressure Increase With Increase In Temperature.
From klatordwr.blob.core.windows.net
Does Pressure Rise With Temperature at Eric Thomson blog Does Pressure Increase With Increase In Temperature When you increase the container volume, you are increasing the degree of freedom of the gas molecules. When the temperature of a system goes up, the pressure also goes up, and vice versa. Temperature also illustrates a direct relationship. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. At constant pressure,. Does Pressure Increase With Increase In Temperature.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Does Pressure Increase With Increase In Temperature When the temperature of a system goes up, the pressure also goes up, and vice versa. Temperature also illustrates a direct relationship. When there is an increase in temperature the kinetic energy of gas molecules increases. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. A graph of pressure vs. Pressure. Does Pressure Increase With Increase In Temperature.
From rect.blob.core.windows.net
The Surprising Relationship How Temperature Influences Conduction Velocity Does Pressure Increase With Increase In Temperature When there is an increase in temperature the kinetic energy of gas molecules increases. When you increase the container volume, you are increasing the degree of freedom of the gas molecules. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. Temperature also illustrates a direct relationship. A graph of pressure. Does Pressure Increase With Increase In Temperature.
From saylordotorg.github.io
Vapor Pressure Does Pressure Increase With Increase In Temperature When you increase the container volume, you are increasing the degree of freedom of the gas molecules. A graph of pressure vs. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. The energy added as work during the compression of a gas leads to an increase in pressure and temperature.. Does Pressure Increase With Increase In Temperature.
From www.savemyexams.co.uk
The Gas Laws (5.3.4) Edexcel IGCSE Physics Revision Notes 2019 Save Does Pressure Increase With Increase In Temperature When there is an increase in temperature the kinetic energy of gas molecules increases. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. Pressure increases with temperature in a closed system with. Does Pressure Increase With Increase In Temperature.
From www.quora.com
If temperature increases, pressure increases. Does temperature increase Does Pressure Increase With Increase In Temperature At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. When you increase the container volume, you are increasing the degree of freedom of the gas molecules. Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of. A graph of pressure vs. Temperature. Does Pressure Increase With Increase In Temperature.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Does Pressure Increase With Increase In Temperature A graph of pressure vs. When you increase the container volume, you are increasing the degree of freedom of the gas molecules. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. Temperature. Does Pressure Increase With Increase In Temperature.
From www.youtube.com
The Effect of Temperature and Pressure on Solubility Mr C YouTube Does Pressure Increase With Increase In Temperature When there is an increase in temperature the kinetic energy of gas molecules increases. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. When the temperature of a system goes up, the pressure also goes up, and vice versa. The energy added as work during the compression of a gas. Does Pressure Increase With Increase In Temperature.
From joifcnqsy.blob.core.windows.net
Does Partial Pressure Increase With Temperature at Linda Adams blog Does Pressure Increase With Increase In Temperature When you increase the container volume, you are increasing the degree of freedom of the gas molecules. A graph of pressure vs. Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. When there. Does Pressure Increase With Increase In Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Pressure Increase With Increase In Temperature A graph of pressure vs. When you increase the container volume, you are increasing the degree of freedom of the gas molecules. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. Temperature also illustrates a direct relationship. When there is an increase in temperature the kinetic energy of gas molecules increases.. Does Pressure Increase With Increase In Temperature.
From brainly.in
diagram of relation between pressure and temperature Brainly.in Does Pressure Increase With Increase In Temperature When the temperature of a system goes up, the pressure also goes up, and vice versa. Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. When you increase the container volume, you. Does Pressure Increase With Increase In Temperature.
From techiescientist.com
Does Density Change With Temperature? Techiescientist Does Pressure Increase With Increase In Temperature At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. A graph of pressure vs. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Temperature also illustrates a direct relationship. When the temperature of a system goes up, the pressure also. Does Pressure Increase With Increase In Temperature.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Does Pressure Increase With Increase In Temperature The energy added as work during the compression of a gas leads to an increase in pressure and temperature. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. A graph of pressure vs. Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy. Does Pressure Increase With Increase In Temperature.
From www.researchgate.net
Variations of the pressure increase, temperature rise, and film Does Pressure Increase With Increase In Temperature When the temperature of a system goes up, the pressure also goes up, and vice versa. Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. When there is an increase in temperature. Does Pressure Increase With Increase In Temperature.
From www.slideshare.net
10.2 First law of Thermodynamics and PV graphs Does Pressure Increase With Increase In Temperature When the temperature of a system goes up, the pressure also goes up, and vice versa. Temperature also illustrates a direct relationship. Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. When you. Does Pressure Increase With Increase In Temperature.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Does Pressure Increase With Increase In Temperature The pressure of a given amount of gas is directly proportional to the temperature at a given volume. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. A graph of pressure vs. Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of.. Does Pressure Increase With Increase In Temperature.
From chart-studio.plotly.com
Water Pressure vs. Temperature line chart made by 18youngt plotly Does Pressure Increase With Increase In Temperature At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. Temperature also illustrates a direct relationship. When you increase the container volume, you are increasing the degree of freedom of the gas molecules. A graph of pressure vs. Pressure increases with temperature in a closed system with constant volume due to. Does Pressure Increase With Increase In Temperature.
From loebantdv.blob.core.windows.net
Why Does Equilibrium Vapor Pressure Increase With Temperature at Thomas Does Pressure Increase With Increase In Temperature At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. Temperature also illustrates a direct relationship. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. A graph of pressure vs. When you increase the container volume, you are increasing the degree. Does Pressure Increase With Increase In Temperature.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Does Pressure Increase With Increase In Temperature The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Temperature also illustrates a direct relationship. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. When you increase the container volume, you are increasing the degree of freedom of the gas molecules.. Does Pressure Increase With Increase In Temperature.
From docslib.org
Relationship Between Density, Pressure, and Temperature DocsLib Does Pressure Increase With Increase In Temperature When there is an increase in temperature the kinetic energy of gas molecules increases. A graph of pressure vs. Temperature also illustrates a direct relationship. Pressure increases with temperature in a closed system with constant volume due to the increased kinetic energy of. When you increase the container volume, you are increasing the degree of freedom of the gas molecules.. Does Pressure Increase With Increase In Temperature.
From klatordwr.blob.core.windows.net
Does Pressure Rise With Temperature at Eric Thomson blog Does Pressure Increase With Increase In Temperature When you increase the container volume, you are increasing the degree of freedom of the gas molecules. The pressure of a given amount of gas is directly proportional to the temperature at a given volume. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Temperature also illustrates a direct relationship.. Does Pressure Increase With Increase In Temperature.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Does Pressure Increase With Increase In Temperature Temperature also illustrates a direct relationship. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. A graph of pressure vs. The pressure of a given amount of gas is directly proportional. Does Pressure Increase With Increase In Temperature.
From www.alamy.com
Charles's law. relationship between volume and temperature. Increasing Does Pressure Increase With Increase In Temperature When you increase the container volume, you are increasing the degree of freedom of the gas molecules. When the temperature of a system goes up, the pressure also goes up, and vice versa. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. The pressure of a given amount of gas. Does Pressure Increase With Increase In Temperature.