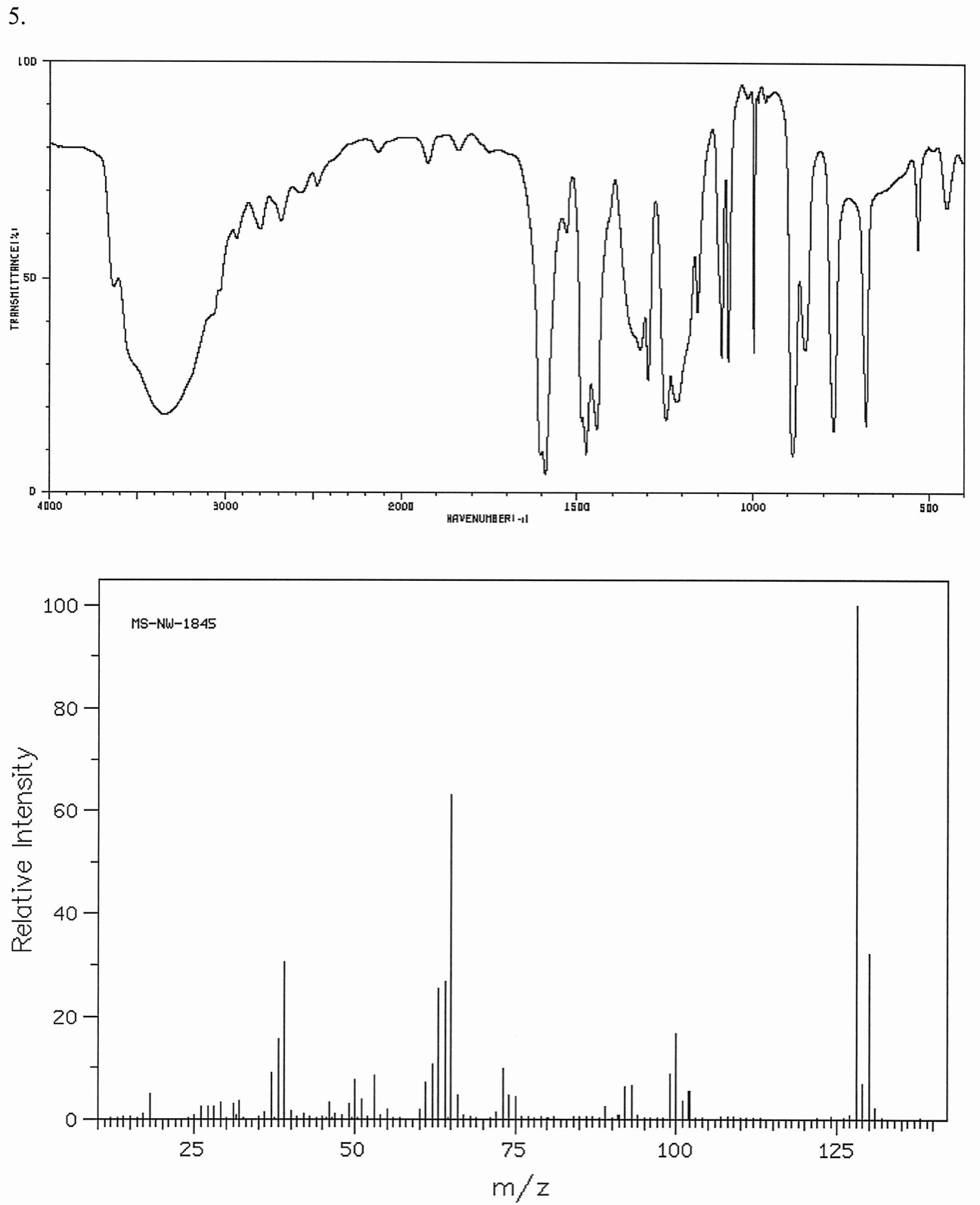
Combined Ir Spectroscopy And Mass Spectrometry Problems . Explain what the fragment for the base peak would be. Match each compound with its corresponding mass spectrum (a,b, and c) and draw the fragment ion corresponding to the base peak in each. Which is the base peak? In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to. Combined ir spectroscopy and mass spectrometry problems. Why is there a peak at 122? Determine the molecular formula and possible structures for each. Which peak represents the m +? Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. An unknown compound gives rise to the. Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra.
from www.chegg.com
In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to. Combined ir spectroscopy and mass spectrometry problems. Which is the base peak? Determine the molecular formula and possible structures for each. Explain what the fragment for the base peak would be. Match each compound with its corresponding mass spectrum (a,b, and c) and draw the fragment ion corresponding to the base peak in each. Why is there a peak at 122? Which peak represents the m +? Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. An unknown compound gives rise to the.
Solved Combined IR Spectroscopy and Mass Spectrometry
Combined Ir Spectroscopy And Mass Spectrometry Problems Explain what the fragment for the base peak would be. Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. Combined ir spectroscopy and mass spectrometry problems. In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to. Match each compound with its corresponding mass spectrum (a,b, and c) and draw the fragment ion corresponding to the base peak in each. Why is there a peak at 122? Which peak represents the m +? Which is the base peak? An unknown compound gives rise to the. In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. Determine the molecular formula and possible structures for each. Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. Explain what the fragment for the base peak would be.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems Combined ir spectroscopy and mass spectrometry problems. Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. Which is the base peak? Why is there a peak at 122? In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to.. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.numerade.com
SOLVED Combined IR Spectroscopy and Mass Spectrometry Problems Combined Ir Spectroscopy And Mass Spectrometry Problems An unknown compound gives rise to the. Which peak represents the m +? In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. Why is there a peak at 122? Match each compound with its corresponding mass spectrum (a,b, and c) and draw the fragment ion corresponding to. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Mass Spectrometry Fragmentation Practice Problem 43 Combined Ir Spectroscopy And Mass Spectrometry Problems Combined ir spectroscopy and mass spectrometry problems. Match each compound with its corresponding mass spectrum (a,b, and c) and draw the fragment ion corresponding to the base peak in each. An unknown compound gives rise to the. In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. Explain. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to. Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. In this post we’re going to go through four (simple) practice problems where you’ll be provided with. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.numerade.com
SOLVED Combined IR Spectroscopy and Mass Spectrometry Problems Combined Ir Spectroscopy And Mass Spectrometry Problems Determine the molecular formula and possible structures for each. In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. Combined ir spectroscopy and mass spectrometry problems.. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved For problems 16 answer the questions on the Mass Combined Ir Spectroscopy And Mass Spectrometry Problems Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. Which is the base peak? Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. In this post we’re going to go through four. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems Which is the base peak? In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to. An unknown compound gives rise to the. Which peak represents the m +? Combined ir spectroscopy and mass spectrometry problems. Explain what the fragment for the base peak would be. In this post we’re going to. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.numerade.com
SOLVED Combined IR Spectroscopy and Mass Spectrometry Problems Combined Ir Spectroscopy And Mass Spectrometry Problems Explain what the fragment for the base peak would be. In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to. Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. Why is there a peak at 122?. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved combined IR spectroscopy and mass spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems Explain what the fragment for the base peak would be. In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. Which is the base peak? An unknown compound gives rise to the. Which peak represents the m +? Combined ir spectroscopy and mass spectrometry problems. Why is there. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to. Which is the base peak? Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. Combined ir and ms problems and answers ** ir, ms and nmr practice exams. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved IR SPECTROSCOPY AND MASS SPECTROMETRY A. On the Combined Ir Spectroscopy And Mass Spectrometry Problems Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems Determine the molecular formula and possible structures for each. An unknown compound gives rise to the. Match each compound with its corresponding mass spectrum (a,b, and c) and draw the fragment ion corresponding to the base peak in each. Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to. Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. Determine the molecular formula and possible structures for each. Match each compound with its corresponding mass spectrum (a,b, and. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems Determine the molecular formula and possible structures for each. Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. Combined ir spectroscopy and mass. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. An unknown compound gives rise to the. Determine the molecular formula and possible structures for each. Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.youtube.com
How to Solve Mass Spectrometry Problems YouTube Combined Ir Spectroscopy And Mass Spectrometry Problems Match each compound with its corresponding mass spectrum (a,b, and c) and draw the fragment ion corresponding to the base peak in each. In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to. Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.studypool.com
SOLUTION Complete mass spectrometry and ir spectroscopy Studypool Combined Ir Spectroscopy And Mass Spectrometry Problems An unknown compound gives rise to the. Which is the base peak? Which peak represents the m +? Combined ir spectroscopy and mass spectrometry problems. In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. Why is there a peak at 122? Match each compound with its corresponding. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.studypool.com
SOLUTION Mass spectroscopy notes principle problems problems Studypool Combined Ir Spectroscopy And Mass Spectrometry Problems In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to. Combined ir spectroscopy and mass spectrometry problems. In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. Explain what the fragment for the base peak would be. Combined ir. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.youtube.com
Mass Spectrometry Practice YouTube Combined Ir Spectroscopy And Mass Spectrometry Problems An unknown compound gives rise to the. Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. Match each compound with its corresponding mass. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. Which is the base peak? In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to. Combined ir spectroscopy and mass spectrometry problems. Match each compound with its corresponding mass. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved C4 на р Ал, Combined IR Spectroscopy and Mass Combined Ir Spectroscopy And Mass Spectrometry Problems In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to. Determine the molecular formula and possible structures for each. Combined ir and ms problems and answers ** ir, ms. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.researchgate.net
Experimental approach of IRIS, combining mass spectrometry with Combined Ir Spectroscopy And Mass Spectrometry Problems Determine the molecular formula and possible structures for each. An unknown compound gives rise to the. Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. Combined ir spectroscopy and mass spectrometry problems. Match each compound with its corresponding mass spectrum (a,b, and c) and draw the fragment. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.studypool.com
SOLUTION Complete mass spectrometry and ir spectroscopy Studypool Combined Ir Spectroscopy And Mass Spectrometry Problems Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. Match each compound with its corresponding mass spectrum (a,b, and c) and draw the fragment ion corresponding to the base peak in each. Combined ir spectroscopy and mass spectrometry problems. In this post we’re going to. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.vrogue.co
Solved Ir And Mass Spectrometry Use The Ir And Ms Spe vrogue.co Combined Ir Spectroscopy And Mass Spectrometry Problems Determine the molecular formula and possible structures for each. In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. Combined ir spectroscopy and mass spectrometry problems. Why is there a peak at 122? Which is the base peak? Combined ir spectroscopy and mass spectrometry problems determine the molecular. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems Match each compound with its corresponding mass spectrum (a,b, and c) and draw the fragment ion corresponding to the base peak in each. Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy). Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. Explain what the fragment for the base peak would be. Which is the base peak? Determine the molecular formula and possible structures for each. An unknown compound gives rise to the. Combined ir and ms problems and answers. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems Why is there a peak at 122? Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. Determine the molecular formula and possible structures for each. Match each compound with its corresponding mass spectrum (a,b, and c) and draw the fragment ion corresponding to the base. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems An unknown compound gives rise to the. Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. Which is the base peak? Determine the molecular formula and possible structures for each. Which peak represents the m +? In this chapter, you will learn about two spectroscopic. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.scribd.com
Chemistry 344 Spectroscopy and Spectrometry Problem Set 1 PDF Mass Combined Ir Spectroscopy And Mass Spectrometry Problems An unknown compound gives rise to the. Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. Determine the molecular formula and possible structures for each. Which is the base peak? Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.numerade.com
SOLVED Combined IR Spectroscopy and Mass Spectrometry Problems Combined Ir Spectroscopy And Mass Spectrometry Problems Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. Which is the base peak? Combined ir spectroscopy and mass spectrometry problems. Match each compound with its corresponding mass spectrum (a,b, and c) and draw the fragment ion corresponding to the base peak in each. Explain. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems An unknown compound gives rise to the. Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. Why is there a peak at 122? Explain what the fragment for the base peak would be. In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems In this post we’re going to go through four (simple) practice problems where you’ll be provided with an ir spectrum and the. Explain what the fragment for the base peak would be. Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. An unknown compound gives rise to. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems Why is there a peak at 122? Combined ir and ms problems and answers ** ir, ms and nmr practice exams 11.10 solving problems using ir and mass spec is shared. Combined ir spectroscopy and mass spectrometry problems. An unknown compound gives rise to the. Which is the base peak? Match each compound with its corresponding mass spectrum (a,b, and. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From www.chegg.com
Solved Combined IR Spectroscopy and Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems Combined ir spectroscopy and mass spectrometry problems. Which is the base peak? Determine the molecular formula and possible structures for each. Which peak represents the m +? In this chapter, you will learn about two spectroscopic techniques (mass spectroscopy and infrared spectroscopy) that are used to. Combined ir and ms problems and answers ** ir, ms and nmr practice exams. Combined Ir Spectroscopy And Mass Spectrometry Problems.
From classfulltotalize.z21.web.core.windows.net
Questions On Mass Spectrometry Combined Ir Spectroscopy And Mass Spectrometry Problems Which is the base peak? Combined ir spectroscopy and mass spectrometry problems determine the molecular formula and possible structures for each unknown based on the given spectra. Match each compound with its corresponding mass spectrum (a,b, and c) and draw the fragment ion corresponding to the base peak in each. In this chapter, you will learn about two spectroscopic techniques. Combined Ir Spectroscopy And Mass Spectrometry Problems.