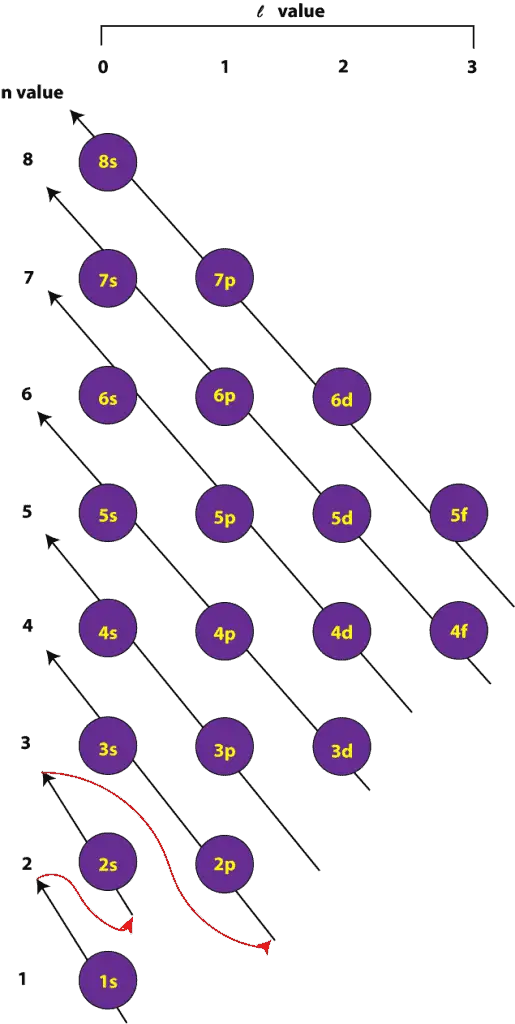
Copper Electron Orbitals . electronic configuration of cu. The electronic configuration of copper (cu) can be represented as: Since 1s can only hold two. to write the orbital diagram for the copper (cu) first we need to write the. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p. in writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling.
from masterconceptsinchemistry.com
electronic configuration of cu. to write the orbital diagram for the copper (cu) first we need to write the. The electronic configuration of copper (cu) can be represented as: if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. Since 1s can only hold two. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p. in writing the electron configuration for copper the first two electrons will go in the 1s orbital.
How’re atomic orbitals filled with electrons?
Copper Electron Orbitals electronic configuration of cu. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p. if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. to write the orbital diagram for the copper (cu) first we need to write the. The electronic configuration of copper (cu) can be represented as: in writing the electron configuration for copper the first two electrons will go in the 1s orbital. Since 1s can only hold two. electronic configuration of cu. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle.
From organicful44.blogspot.com
copper orbital diagram Organicful Copper Electron Orbitals to write the orbital diagram for the copper (cu) first we need to write the. in writing the electron configuration for copper the first two electrons will go in the 1s orbital. Since 1s can only hold two. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. Copper’s. Copper Electron Orbitals.
From chemistry.stackexchange.com
electronic configuration If copper has 2 valance electrons and sulfur Copper Electron Orbitals in writing the electron configuration for copper the first two electrons will go in the 1s orbital. to write the orbital diagram for the copper (cu) first we need to write the. if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. electronic configuration of cu. The electronic. Copper Electron Orbitals.
From valenceelectrons.com
Copper(Cu) electron configuration and orbital diagram Copper Electron Orbitals The electronic configuration of copper (cu) can be represented as: Since 1s can only hold two. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. electronic configuration of cu. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. in the copper orbital diagram, the 1s subshell. Copper Electron Orbitals.
From brainly.in
Why valency of Scandium is 3 but in google electronic configuration is Copper Electron Orbitals in writing the electron configuration for copper the first two electrons will go in the 1s orbital. to write the orbital diagram for the copper (cu) first we need to write the. Since 1s can only hold two. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. The electronic configuration of copper (cu) can be represented. Copper Electron Orbitals.
From chem.libretexts.org
Introduction to Crystal Field Theory Chemistry LibreTexts Copper Electron Orbitals cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. The electronic configuration of copper (cu) can be represented as: Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. Since 1s can only hold two. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s. Copper Electron Orbitals.
From mungfali.com
Periodic Table With Orbitals Labeled Copper Electron Orbitals to write the orbital diagram for the copper (cu) first we need to write the. electronic configuration of cu. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. The electronic configuration of copper (cu) can be represented as: in writing the electron configuration for copper the first two electrons will go in the 1s orbital.. Copper Electron Orbitals.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Electron Orbitals The electronic configuration of copper (cu) can be represented as: cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. Since 1s can only hold two. electronic configuration of cu. in writing the electron configuration for copper the first two electrons will go in the 1s orbital. to. Copper Electron Orbitals.
From www.alamy.es
3D Render de la estructura del átomo de cobre aislado sobre fondo Copper Electron Orbitals in writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. The electronic configuration of copper (cu) can be represented as: if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. in. Copper Electron Orbitals.
From valenceelectrons.com
How to Find the Valence Electrons for Copper (Cu)? Copper Electron Orbitals cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. The electronic configuration of copper (cu) can be represented as: in writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. to. Copper Electron Orbitals.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry Copper Electron Orbitals to write the orbital diagram for the copper (cu) first we need to write the. electronic configuration of cu. if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. in writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper’s electron. Copper Electron Orbitals.
From www.vectorstock.com
Symbol and electron diagram for copper Royalty Free Vector Copper Electron Orbitals in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p. in writing the electron configuration for copper the first two electrons will go in the 1s orbital. Since 1s can only hold two. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according. Copper Electron Orbitals.
From valenceelectrons.com
How to Find the Valence Electrons for Copper (Cu)? Copper Electron Orbitals to write the orbital diagram for the copper (cu) first we need to write the. Since 1s can only hold two. The electronic configuration of copper (cu) can be represented as: in writing the electron configuration for copper the first two electrons will go in the 1s orbital. cu has 4 electronic levels and 3 orbitals based. Copper Electron Orbitals.
From ar.inspiredpencil.com
Copper Orbital Diagram Copper Electron Orbitals Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. electronic configuration of cu. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p. Since 1s can only hold. Copper Electron Orbitals.
From www.technocrazed.com
Principal quantum number n and maximum number of electrons per shell Copper Electron Orbitals if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p. Since 1s can only hold two. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells. Copper Electron Orbitals.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration Copper Electron Orbitals if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. to write the orbital diagram for the copper (cu) first we need to write the. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p. in writing the electron. Copper Electron Orbitals.
From socratic.org
What is a set of four quantum numbers that could represent the last Copper Electron Orbitals Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. electronic configuration of cu. The electronic configuration of copper. Copper Electron Orbitals.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electron Orbitals Since 1s can only hold two. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p. if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. electronic configuration of cu. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron. Copper Electron Orbitals.
From masterconceptsinchemistry.com
How’re atomic orbitals filled with electrons? Copper Electron Orbitals Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. The electronic configuration of copper (cu) can be represented as: Since 1s can only hold two. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s. Copper Electron Orbitals.
From www.mooramo.com
Ions of Transition Elements Mooramo Copper Electron Orbitals electronic configuration of cu. to write the orbital diagram for the copper (cu) first we need to write the. in writing the electron configuration for copper the first two electrons will go in the 1s orbital. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. Since 1s. Copper Electron Orbitals.
From favpng.com
Bohr Model Atom Copper Electron Shell Diagram, PNG, 658x681px, Bohr Copper Electron Orbitals electronic configuration of cu. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. The electronic configuration of copper (cu) can be represented as: if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. to write the orbital diagram for. Copper Electron Orbitals.
From diagrampartkappel.z13.web.core.windows.net
No2 Molecular Orbital Diagram Copper Electron Orbitals to write the orbital diagram for the copper (cu) first we need to write the. if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. electronic configuration of cu. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p.. Copper Electron Orbitals.
From shaunmwilliams.com
Lecture 7 Presentation Copper Electron Orbitals Since 1s can only hold two. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. to write the orbital diagram for the copper (cu) first we need to write. Copper Electron Orbitals.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Electron Orbitals Since 1s can only hold two. to write the orbital diagram for the copper (cu) first we need to write the. The electronic configuration of copper (cu) can be represented as: Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to. Copper Electron Orbitals.
From ar.inspiredpencil.com
Electron Configuration Diagram Orbitals Copper Electron Orbitals if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. Since 1s can only hold two. The electronic configuration of copper (cu) can be represented as: in the copper orbital diagram,. Copper Electron Orbitals.
From fity.club
Orbital Diagram For Copper Copper Electron Orbitals in writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. to write the orbital diagram for the copper (cu) first we need to write the. electronic configuration of cu. if the general pattern of filling electron orbitals is. Copper Electron Orbitals.
From fity.club
Orbital Diagram For Copper Copper Electron Orbitals The electronic configuration of copper (cu) can be represented as: in writing the electron configuration for copper the first two electrons will go in the 1s orbital. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p. Since 1s can only hold two. electronic configuration of cu. . Copper Electron Orbitals.
From pubs.acs.org
Insight into the Effect of the dOrbital Energy of Copper Ions in Metal Copper Electron Orbitals if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. electronic configuration of cu. The electronic configuration of copper (cu) can be represented as: in writing the electron configuration for. Copper Electron Orbitals.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper Electron Orbitals electronic configuration of cu. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. to write the orbital diagram for the copper (cu) first we need to write the. The electronic configuration of copper (cu) can be represented. Copper Electron Orbitals.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Electron Orbitals to write the orbital diagram for the copper (cu) first we need to write the. if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. electronic configuration of cu. in writing the electron configuration for copper the. Copper Electron Orbitals.
From mungfali.com
Orbital Subshell Chart Copper Electron Orbitals The electronic configuration of copper (cu) can be represented as: electronic configuration of cu. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p. if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. Since 1s can only hold two.. Copper Electron Orbitals.
From chem.libretexts.org
1.6 Atomic Orbitals A Quantum Mechanical Description of Electrons Copper Electron Orbitals if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. in the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p. Since 1s. Copper Electron Orbitals.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science Copper Electron Orbitals Since 1s can only hold two. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. in writing the electron configuration for copper the first two electrons will go in the 1s orbital. The electronic configuration of copper (cu). Copper Electron Orbitals.
From www.mooramo.com
Gaining and Losing Electrons Mooramo Copper Electron Orbitals in writing the electron configuration for copper the first two electrons will go in the 1s orbital. to write the orbital diagram for the copper (cu) first we need to write the. cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. electronic configuration of cu. The electronic. Copper Electron Orbitals.
From socratic.org
What are the different kinds of f orbitals? Socratic Copper Electron Orbitals cu has 4 electronic levels and 3 orbitals based on increasing energy of shells according to aufbau principle. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. electronic configuration of cu. The electronic configuration of copper (cu) can be represented as: Since 1s can only hold two. in the copper orbital diagram, the 1s subshell. Copper Electron Orbitals.
From favpng.com
Bohr Model Atom Electron Shell Copper, PNG, 512x512px, Bohr Model, Area Copper Electron Orbitals in writing the electron configuration for copper the first two electrons will go in the 1s orbital. if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. cu has 4 electronic levels and 3 orbitals based on increasing. Copper Electron Orbitals.