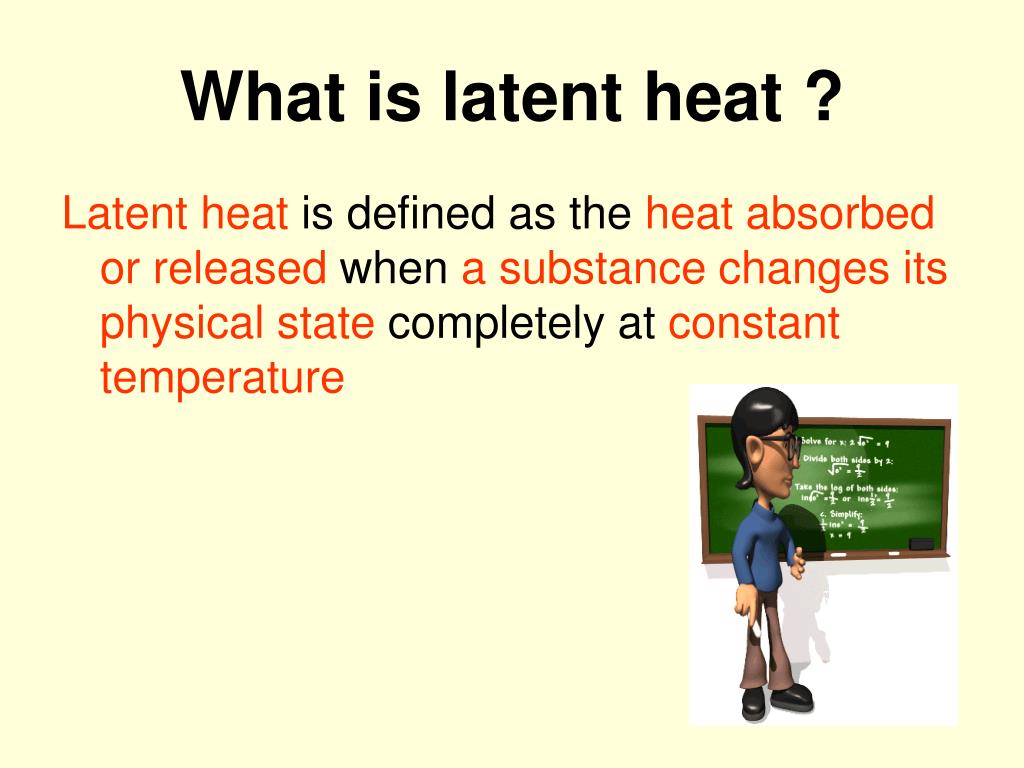
Heat Definition Chemistry . heat, energy that is transferred from one body to another as the result of a difference in temperature. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. heat flows from a higher temperature to a lower temperature. Unlike thermal energy, heat is not a property of the substance. define heat and work, and describe an important limitation in their interconversion. Describe the physical meaning of. heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. Heat is the thermal energy transfer between systems or bodies due to a temperature difference.
from www.slideserve.com
define heat and work, and describe an important limitation in their interconversion. heat flows from a higher temperature to a lower temperature. heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. Describe the physical meaning of. Unlike thermal energy, heat is not a property of the substance. heat, energy that is transferred from one body to another as the result of a difference in temperature. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. Heat is the thermal energy transfer between systems or bodies due to a temperature difference.
PPT 4.3 SPECIFIC LATENT HEAT PowerPoint Presentation, free download
Heat Definition Chemistry Unlike thermal energy, heat is not a property of the substance. define heat and work, and describe an important limitation in their interconversion. Unlike thermal energy, heat is not a property of the substance. Describe the physical meaning of. heat flows from a higher temperature to a lower temperature. heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat is the thermal energy transfer between systems or bodies due to a temperature difference.
From www.teachoo.com
For any substance, why does the temperature remain constant during the Heat Definition Chemistry Heat is the thermal energy transfer between systems or bodies due to a temperature difference. heat flows from a higher temperature to a lower temperature. define heat and work, and describe an important limitation in their interconversion. Describe the physical meaning of. heat is energy that is transferred from one object or substance to another because of. Heat Definition Chemistry.
From samson-jolpblogsantos.blogspot.com
How Does Temperature Affect Solids Liquids and Gases Heat Definition Chemistry heat flows from a higher temperature to a lower temperature. Describe the physical meaning of. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. Unlike thermal energy, heat is not. Heat Definition Chemistry.
From definitionxc.blogspot.com
Molar Heat Of Fusion Definition Chemistry DEFINITIONXC Heat Definition Chemistry heat, energy that is transferred from one body to another as the result of a difference in temperature. heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the. Heat Definition Chemistry.
From fity.club
Specific Heat Heat Definition Chemistry heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. heat, energy that is transferred from one body to another as the result of a difference in temperature. heat flows from a higher temperature to a lower temperature. define heat and work, and describe an. Heat Definition Chemistry.
From www.slideserve.com
PPT Heat and Temperature PowerPoint Presentation, free download ID Heat Definition Chemistry Unlike thermal energy, heat is not a property of the substance. heat flows from a higher temperature to a lower temperature. define heat and work, and describe an important limitation in their interconversion. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. Heat is. Heat Definition Chemistry.
From mavink.com
Formula Of Specific Heat Capacity Heat Definition Chemistry Describe the physical meaning of. define heat and work, and describe an important limitation in their interconversion. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. heat flows from a higher temperature to a lower temperature. Unlike thermal energy, heat is not a property of the substance. the heat capacity is. Heat Definition Chemistry.
From www.ck12.org
Heats of Vaporization and Condensation CK12 Foundation Heat Definition Chemistry heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. define heat and work, and describe an important limitation in their interconversion. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Unlike thermal energy, heat is not a property of the. Heat Definition Chemistry.
From azchemistry.com
20 Heat Transfer Through Conduction Example in Daily Life AZ Chemistry Heat Definition Chemistry the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. define heat and work, and describe an important limitation in their interconversion. Describe the physical meaning of. heat flows from a higher temperature to a lower temperature. Heat is the thermal energy transfer between systems. Heat Definition Chemistry.
From www.sliderbase.com
Heat Presentation Chemistry Heat Definition Chemistry Heat is the thermal energy transfer between systems or bodies due to a temperature difference. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. Describe the physical meaning of. heat flows from a higher temperature to a lower temperature. Unlike thermal energy, heat is not. Heat Definition Chemistry.
From prushaann.blogspot.com
Chemical Energy Physics Definition Physics for Kids Energy An easy Heat Definition Chemistry heat, energy that is transferred from one body to another as the result of a difference in temperature. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. heat flows from a higher temperature to a lower temperature. Describe the physical meaning of. heat. Heat Definition Chemistry.
From www.slideserve.com
PPT Thermal Properties of Matter PowerPoint Presentation, free Heat Definition Chemistry heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. define heat and work, and describe an important limitation in their interconversion. heat, energy that is transferred from one body to another as the result of a difference in temperature. the heat capacity is the. Heat Definition Chemistry.
From ashlynn-has-chang.blogspot.com
Which of the Following Statements Correctly Describe Specific Heat Heat Definition Chemistry the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. Describe the physical meaning of. define heat and work, and describe an important limitation in their interconversion. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Unlike thermal energy, heat. Heat Definition Chemistry.
From www.vrogue.co
Difference Between Oxidation And Combustion Differenc vrogue.co Heat Definition Chemistry Unlike thermal energy, heat is not a property of the substance. heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. heat, energy that is transferred from one body to another as. Heat Definition Chemistry.
From www.slideserve.com
PPT Thermochemistry The heat energy of chemical reactions PowerPoint Heat Definition Chemistry Heat is the thermal energy transfer between systems or bodies due to a temperature difference. define heat and work, and describe an important limitation in their interconversion. heat, energy that is transferred from one body to another as the result of a difference in temperature. Unlike thermal energy, heat is not a property of the substance. heat. Heat Definition Chemistry.
From sciencenotes.org
What Is Temperature? Definition in Science Heat Definition Chemistry the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. heat, energy that is transferred from one body to another as the result of. Heat Definition Chemistry.
From ar.inspiredpencil.com
Heat Definition Heat Definition Chemistry heat, energy that is transferred from one body to another as the result of a difference in temperature. define heat and work, and describe an important limitation in their interconversion. Describe the physical meaning of. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree.. Heat Definition Chemistry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3170116 Heat Definition Chemistry define heat and work, and describe an important limitation in their interconversion. Unlike thermal energy, heat is not a property of the substance. heat, energy that is transferred from one body to another as the result of a difference in temperature. heat flows from a higher temperature to a lower temperature. Describe the physical meaning of. . Heat Definition Chemistry.
From davida.davivienda.com
Specific Heat And Heat Capacity Worksheet Answers Printable Word Searches Heat Definition Chemistry heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. heat, energy that is transferred from one body to another as the result of. Heat Definition Chemistry.
From animalia-life.club
Examples Of Heat Energy Heat Definition Chemistry Describe the physical meaning of. define heat and work, and describe an important limitation in their interconversion. heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. Unlike thermal energy, heat is not a property of the substance. Heat is the thermal energy transfer between systems or. Heat Definition Chemistry.
From mahadanaqabil.blogspot.com
2nd Thermodynamics Mahadana Qabil Heat Definition Chemistry heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. heat, energy that is transferred from one body to another as the result of a difference in temperature. Unlike thermal energy, heat is not a property of the substance. the heat capacity is the amount of. Heat Definition Chemistry.
From guides.co
States of Matter FD202 Fundamentals of Fire and Combustion on Guides Heat Definition Chemistry heat flows from a higher temperature to a lower temperature. Unlike thermal energy, heat is not a property of the substance. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. define heat and work, and describe an important limitation in their interconversion. heat, energy that is transferred from one body to. Heat Definition Chemistry.
From www.expii.com
Vapor Pressure — Definition & Overview Expii Heat Definition Chemistry Heat is the thermal energy transfer between systems or bodies due to a temperature difference. heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. define heat and work, and describe an important limitation in their interconversion. the heat capacity is the amount of heat, expressed. Heat Definition Chemistry.
From www.yourdictionary.com
Difference Between Heat and Temperature in Simple Terms YourDictionary Heat Definition Chemistry Unlike thermal energy, heat is not a property of the substance. heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Describe the physical meaning of. the heat capacity is the amount of heat, expressed. Heat Definition Chemistry.
From www.slideserve.com
PPT 4.3 SPECIFIC LATENT HEAT PowerPoint Presentation, free download Heat Definition Chemistry heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. define heat and. Heat Definition Chemistry.
From study.com
Heat of Fusion Definition, Equation & Examples Video & Lesson Heat Definition Chemistry heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. Heat is the thermal energy transfer between systems or bodies due to a temperature difference.. Heat Definition Chemistry.
From courses.lumenlearning.com
Specific Heat Boundless Physics Heat Definition Chemistry heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. heat flows from a higher temperature to a lower temperature. Describe the physical meaning of. the heat capacity is the amount of heat, expressed. Heat Definition Chemistry.
From meaningkosh.com
What Is Heat Of Formation MeaningKosh Heat Definition Chemistry Unlike thermal energy, heat is not a property of the substance. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat is the thermal energy transfer between. Heat Definition Chemistry.
From studyonline.blog
What Is Chemical Energy? Definition and Examples Heat Definition Chemistry Unlike thermal energy, heat is not a property of the substance. heat flows from a higher temperature to a lower temperature. heat, energy that is transferred from one body to another as the result of a difference in temperature. Describe the physical meaning of. Heat is the thermal energy transfer between systems or bodies due to a temperature. Heat Definition Chemistry.
From quizlet.com
Heat Transfer Diagram Quizlet Heat Definition Chemistry heat, energy that is transferred from one body to another as the result of a difference in temperature. define heat and work, and describe an important limitation in their interconversion. Unlike thermal energy, heat is not a property of the substance. heat flows from a higher temperature to a lower temperature. Describe the physical meaning of. . Heat Definition Chemistry.
From www.sciencefacts.net
Heat Transfer Definition, Types, And Examples Heat Definition Chemistry heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. Describe the physical meaning of. define heat and work, and describe an important limitation in their interconversion. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. heat flows from a. Heat Definition Chemistry.
From www.jlcatj.gob.mx
Econo Heat Discount Offers, Save 60 jlcatj.gob.mx Heat Definition Chemistry heat, energy that is transferred from one body to another as the result of a difference in temperature. heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. define heat and work, and describe an important limitation in their interconversion. the heat capacity is the. Heat Definition Chemistry.
From www.chemistrylearner.com
Heat (Enthalpy) of Solution Definition, Formula, & Problems Heat Definition Chemistry heat, energy that is transferred from one body to another as the result of a difference in temperature. heat flows from a higher temperature to a lower temperature. define heat and work, and describe an important limitation in their interconversion. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to. Heat Definition Chemistry.
From gamesmartz.com
Heat Definition & Image GameSmartz Heat Definition Chemistry Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Unlike thermal energy, heat is not a property of the substance. define heat and work, and describe an important limitation in their interconversion. Describe the physical meaning of. heat flows from a higher temperature to a lower temperature. heat, energy that is. Heat Definition Chemistry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID1281784 Heat Definition Chemistry Unlike thermal energy, heat is not a property of the substance. heat, energy that is transferred from one body to another as the result of a difference in temperature. the heat capacity is the amount of heat, expressed usually in joules or calories, needed to change the system by 1 degree. heat flows from a higher temperature. Heat Definition Chemistry.
From www.pinterest.com
Specific Heat Easy Science Teaching chemistry, Sensible heat Heat Definition Chemistry heat is energy that is transferred from one object or substance to another because of a difference in temperature between them. heat, energy that is transferred from one body to another as the result of a difference in temperature. heat flows from a higher temperature to a lower temperature. Heat is the thermal energy transfer between systems. Heat Definition Chemistry.