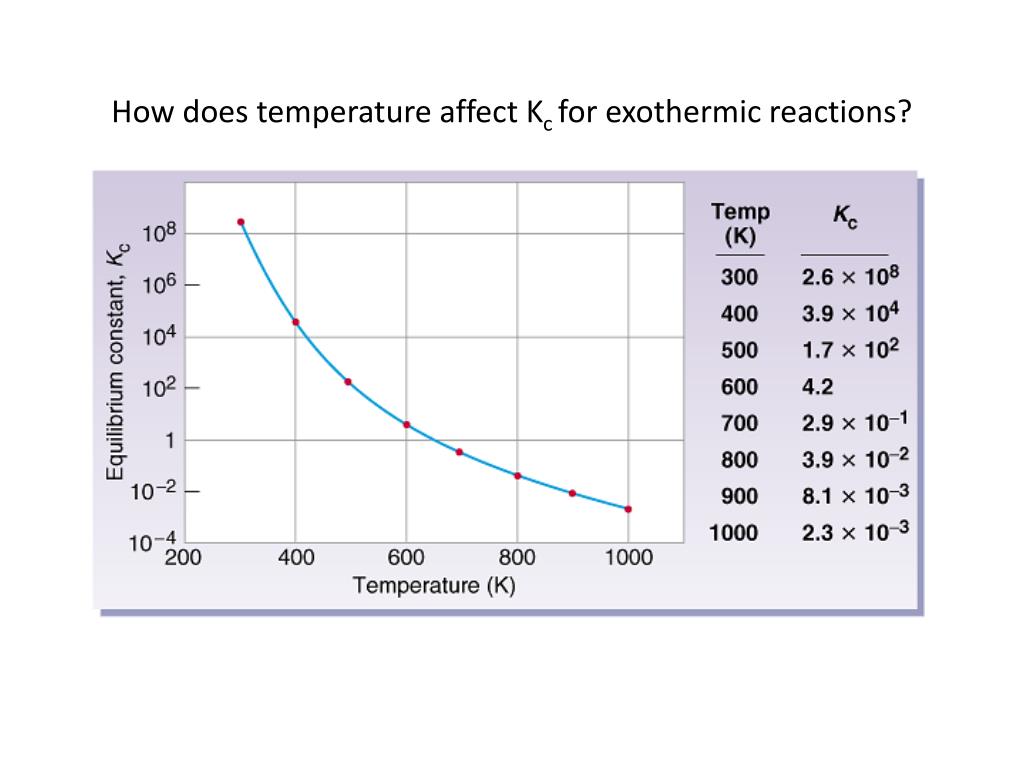
How Does Temperature Affect Ksp . It depends on temperature and solvation enthalpy of ions. The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form, and. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. Learn how to calculate ksp and its. In general, as temperature increases, the molar solubility of most compounds also increases. Ksp is the equilibrium constant for the dissolution of a solid substance in water. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. Whereas solubility is usually expressed in terms of. Temperature can affect the molar solubility of a compound in a solution.
from www.slideserve.com
\(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form, and. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. In general, as temperature increases, the molar solubility of most compounds also increases. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. Learn how to calculate ksp and its. Temperature can affect the molar solubility of a compound in a solution. Ksp is the equilibrium constant for the dissolution of a solid substance in water. It depends on temperature and solvation enthalpy of ions. Whereas solubility is usually expressed in terms of.
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review
How Does Temperature Affect Ksp Whereas solubility is usually expressed in terms of. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. Learn how to calculate ksp and its. Temperature can affect the molar solubility of a compound in a solution. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. It depends on temperature and solvation enthalpy of ions. Ksp is the equilibrium constant for the dissolution of a solid substance in water. Whereas solubility is usually expressed in terms of. The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form, and. In general, as temperature increases, the molar solubility of most compounds also increases. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound.
From www.youtube.com
KSP temperature measurement in area Delta YouTube How Does Temperature Affect Ksp Temperature can affect the molar solubility of a compound in a solution. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. In general, as temperature increases, the molar solubility of most compounds also increases. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of. How Does Temperature Affect Ksp.
From slideplayer.com
UNIT III Tutorial 10 Ksp Calculations ppt download How Does Temperature Affect Ksp The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting. How Does Temperature Affect Ksp.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review How Does Temperature Affect Ksp The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. Temperature can affect the molar solubility of a compound in a solution. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. The solubility product constant, ksp. How Does Temperature Affect Ksp.
From www.reddit.com
Relationship between ambient temperature and atmospheric entry heating How Does Temperature Affect Ksp The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form, and. Temperature can affect the molar solubility of a compound in a solution. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. Whereas solubility is usually. How Does Temperature Affect Ksp.
From www.youtube.com
Effect of Temperature on Equilibrium Constants (Extension Material How Does Temperature Affect Ksp Temperature can affect the molar solubility of a compound in a solution. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form, and. Learn how to calculate. How Does Temperature Affect Ksp.
From www.youtube.com
Using the Oberth Effect KSP (Not) Beginner's Guide YouTube How Does Temperature Affect Ksp Whereas solubility is usually expressed in terms of. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. Learn how to calculate ksp and its. Temperature can affect the. How Does Temperature Affect Ksp.
From www.researchgate.net
The effects of temperature on the catalase activity in the tissues of How Does Temperature Affect Ksp Ksp is the equilibrium constant for the dissolution of a solid substance in water. In general, as temperature increases, the molar solubility of most compounds also increases. Temperature can affect the molar solubility of a compound in a solution. It depends on temperature and solvation enthalpy of ions. The equilibrium constant for a dissolution reaction, called the solubility product (ksp),. How Does Temperature Affect Ksp.
From www.acs.org
Simulations & Videos for Lesson 5.6 Does Temperature Affect Dissolving How Does Temperature Affect Ksp The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form, and. Ksp is the equilibrium constant for the dissolution of a solid substance in water. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on. How Does Temperature Affect Ksp.
From www.numerade.com
VIDEO solution The circular motion increases with the observed value How Does Temperature Affect Ksp \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. Temperature can affect the molar solubility of a compound in a solution. Learn how to calculate ksp and its.. How Does Temperature Affect Ksp.
From www.numerade.com
SOLVED How does temperature affect the solubility and Ksp of Ca(IO3)2 How Does Temperature Affect Ksp The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form, and. Ksp is the equilibrium constant for the dissolution of a solid substance in water. Temperature can affect the molar solubility of a compound in a solution. \(k_{sq}\) is defined in terms of activity rather than. How Does Temperature Affect Ksp.
From www.numerade.com
SOLVED Using the graph of solubility of common salts as a function of How Does Temperature Affect Ksp \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. Temperature can affect the molar solubility of a compound in a solution. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. Whereas solubility is usually expressed in terms. How Does Temperature Affect Ksp.
From www.chegg.com
Solved Use the class temperature and Ksp values to make an How Does Temperature Affect Ksp Temperature can affect the molar solubility of a compound in a solution. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. Whereas solubility is usually expressed. How Does Temperature Affect Ksp.
From www.numerade.com
SOLVED How does temperature affect the solubility of a salt? Is it How Does Temperature Affect Ksp Learn how to calculate ksp and its. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. Whereas solubility is usually expressed in terms of. Temperature can affect the molar solubility of a compound in a solution. Ksp is the equilibrium constant for the dissolution of a solid substance in water.. How Does Temperature Affect Ksp.
From www.youtube.com
TEMPERATURE MEASUREMENTS Let's Play KSP 1.1 Career Mode Ep.5 How Does Temperature Affect Ksp Ksp is the equilibrium constant for the dissolution of a solid substance in water. Temperature can affect the molar solubility of a compound in a solution. Whereas solubility is usually expressed in terms of. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. In general, as temperature. How Does Temperature Affect Ksp.
From www.slideserve.com
PPT how does temperature effect total energy consumption? PowerPoint How Does Temperature Affect Ksp Learn how to calculate ksp and its. The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form, and. In general, as temperature increases, the molar solubility of most compounds also increases. Ksp is the equilibrium constant for the dissolution of a solid substance in water. It. How Does Temperature Affect Ksp.
From www.studocu.com
Module 3 Lab Solubility and Ksp PART 1) AgCl Temperature (oC) Volume How Does Temperature Affect Ksp The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. Temperature can affect the molar solubility of a compound in a solution. Ksp is the equilibrium constant for the dissolution of a solid substance in water. The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important. How Does Temperature Affect Ksp.
From www.youtube.com
KSP Mission Take temperature reading on location at surface of Kerbin How Does Temperature Affect Ksp Temperature can affect the molar solubility of a compound in a solution. Learn how to calculate ksp and its. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of. How Does Temperature Affect Ksp.
From www.chegg.com
B. Determine Temperature Dependence of Ksp* This will How Does Temperature Affect Ksp \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. Whereas solubility is usually expressed in terms of. It depends on temperature and solvation enthalpy of ions. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound.. How Does Temperature Affect Ksp.
From klaqbwljy.blob.core.windows.net
Why Does Ksp Decrease With Temperature at Mary Doyal blog How Does Temperature Affect Ksp The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. Learn how to calculate ksp and its. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. Whereas solubility is usually expressed in terms of. It depends. How Does Temperature Affect Ksp.
From quizdbpharmacies.z4.web.core.windows.net
How Does Temperature Affect Matter How Does Temperature Affect Ksp \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. It depends on temperature and solvation enthalpy of ions. Learn how to calculate ksp and its. Ksp is the equilibrium constant for the dissolution of a solid substance in water. The equilibrium constant for a dissolution reaction, called. How Does Temperature Affect Ksp.
From www.researchgate.net
ln(Ksp) vs temperature for MgSO4‚7H2O(s). The bold line represents the How Does Temperature Affect Ksp Whereas solubility is usually expressed in terms of. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. Temperature can affect the molar solubility of a compound in a solution. It depends on temperature and solvation enthalpy of ions. The solubility constant can be affected by temperature, pressure, and. How Does Temperature Affect Ksp.
From www.researchgate.net
Determination of Ksp, log Ksp, ∆G, ∆H and ∆S at different... Download How Does Temperature Affect Ksp Whereas solubility is usually expressed in terms of. The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form, and. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. \(k_{sq}\) is defined in terms of. How Does Temperature Affect Ksp.
From slideplayer.com
Ksp and Solubility Equilibria ppt download How Does Temperature Affect Ksp Whereas solubility is usually expressed in terms of. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. Ksp is the equilibrium constant for the dissolution of a solid substance in water. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an. How Does Temperature Affect Ksp.
From www.researchgate.net
Determination of Ksp, log Ksp, ∆G, ∆H and ∆S at different... Download How Does Temperature Affect Ksp The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. It depends on temperature and solvation enthalpy of ions. Temperature can affect the molar solubility of a compound in a solution. In general, as temperature increases, the molar solubility of most compounds also increases. Whereas solubility is usually expressed in terms. How Does Temperature Affect Ksp.
From www.coltsteele.com
Understanding OpenAI's Temperature Parameter Colt Steele How Does Temperature Affect Ksp The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. Ksp is the equilibrium constant for the dissolution of a solid substance in water. The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form, and.. How Does Temperature Affect Ksp.
From slideplayer.com
S as an energy relationship ppt download How Does Temperature Affect Ksp Whereas solubility is usually expressed in terms of. Ksp is the equilibrium constant for the dissolution of a solid substance in water. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. Temperature can affect the molar solubility of a compound in a solution. It depends on temperature. How Does Temperature Affect Ksp.
From github.com
GitHub KSPRO/RealHeat A real heating (not temperature) model for KSP How Does Temperature Affect Ksp Ksp is the equilibrium constant for the dissolution of a solid substance in water. Temperature can affect the molar solubility of a compound in a solution. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. It depends on temperature and solvation enthalpy of ions. Learn how to calculate ksp and. How Does Temperature Affect Ksp.
From www.numerade.com
SOLVED How does temperature affect the solubility of a salt? Is it How Does Temperature Affect Ksp The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. In general, as temperature increases, the molar solubility of most compounds also increases. The solubility constant can be affected. How Does Temperature Affect Ksp.
From chart-studio.plotly.com
Relationship Between 1/Temperature (Kelvin) and Ln(Ksp) scatter chart How Does Temperature Affect Ksp The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form, and. Ksp is the equilibrium constant for the dissolution of a solid substance in water. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on. How Does Temperature Affect Ksp.
From chart-studio.plotly.com
Ln(Ksp) vs. Reciprocal of Absolute Temperature scatter chart made by How Does Temperature Affect Ksp The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if. How Does Temperature Affect Ksp.
From www.slideserve.com
PPT How does temperature effect ectotherms ? PowerPoint Presentation How Does Temperature Affect Ksp Learn how to calculate ksp and its. The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form, and. Whereas solubility is usually expressed in terms of. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a. How Does Temperature Affect Ksp.
From klaqbwljy.blob.core.windows.net
Why Does Ksp Decrease With Temperature at Mary Doyal blog How Does Temperature Affect Ksp Ksp is the equilibrium constant for the dissolution of a solid substance in water. In general, as temperature increases, the molar solubility of most compounds also increases. The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form, and. The equilibrium constant for a dissolution reaction, called. How Does Temperature Affect Ksp.
From www.youtube.com
Effect of Temperature on Solubility // HSC Chemistry YouTube How Does Temperature Affect Ksp It depends on temperature and solvation enthalpy of ions. Whereas solubility is usually expressed in terms of. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. The solubility product constant, ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. Learn how to. How Does Temperature Affect Ksp.
From slidetodoc.com
Chemical Equilibrium Ksp of CaOH2 Equilibrium n Reaction How Does Temperature Affect Ksp \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. Ksp is the equilibrium constant for the dissolution of a solid substance in water. The solubility constant can be affected by temperature, pressure, and molecular size, and it’s important for determining solubility, predicting if a precipitate will form,. How Does Temperature Affect Ksp.
From gaming.stackexchange.com
kerbal space program How does the temperature overlay work? Arqade How Does Temperature Affect Ksp It depends on temperature and solvation enthalpy of ions. Ksp is the equilibrium constant for the dissolution of a solid substance in water. \(k_{sq}\) is defined in terms of activity rather than concentration because it is a measure of a concentration that depends on certain. Whereas solubility is usually expressed in terms of. In general, as temperature increases, the molar. How Does Temperature Affect Ksp.