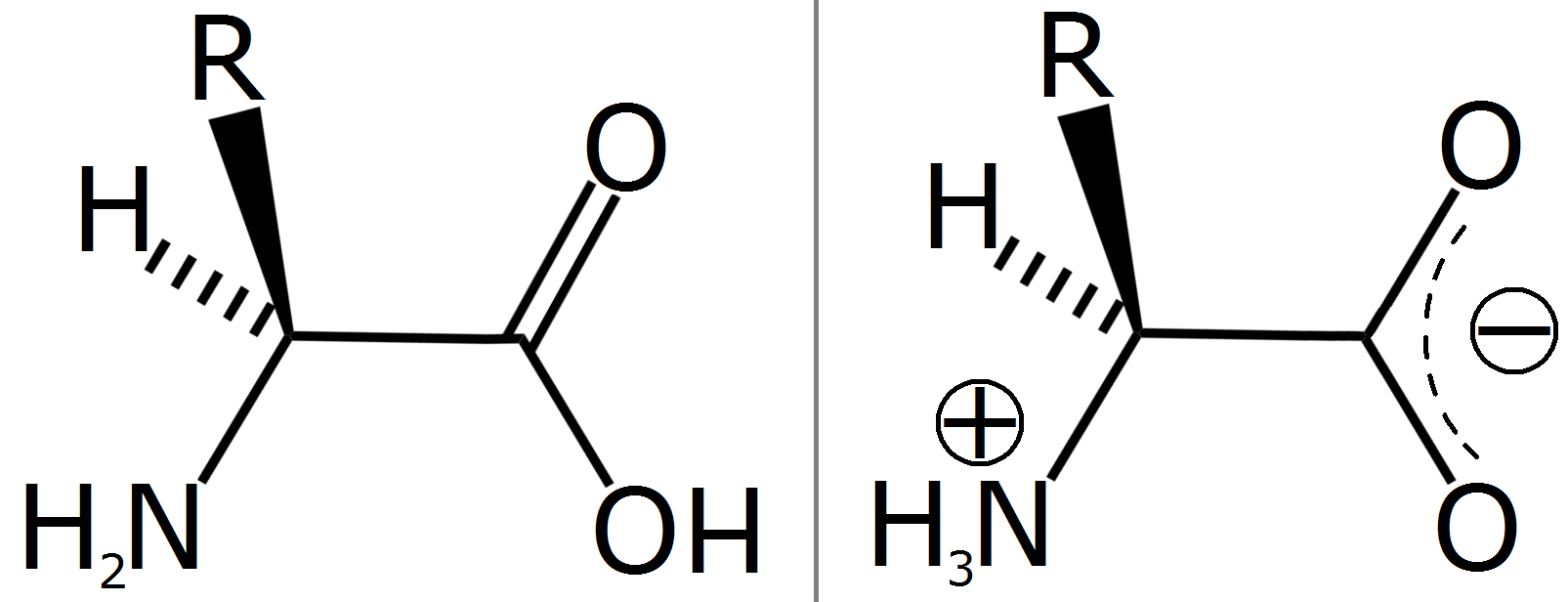
Amino Acids Zwitterion . They are made up of an ammonium or amino group which contains a positive charge. Amino acids are examples of zwitterions. Amino acids are the most common example of zwitterions. At ph values above or below the isoelectric. Draw the zwitterion form of a given amino acid. The charged functional groups connect to the rest of the molecule by covalent bonds. Thus, amino acids exist in aqueous solution primarily in the form of a dipolar ion, or zwitterion (from the german zwitter, meaning “hybrid”). Amino acids are therefore soluble crystalline solids. An amino acid molecule can interact within itself to form a zwitterion. In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged functional groups. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Each amino acid has its own pi value based on the properties of the amino acid. Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids. Account for some of the typical properties of amino acids (e.g., high melting points, solubility. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge.
from es-academic.com
Amino acids are examples of zwitterions. Thus, amino acids exist in aqueous solution primarily in the form of a dipolar ion, or zwitterion (from the german zwitter, meaning “hybrid”). Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids. At ph values above or below the isoelectric. They are made up of an ammonium or amino group which contains a positive charge. Amino acids are the most common example of zwitterions. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. An amino acid molecule can interact within itself to form a zwitterion. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged functional groups.
Zwitterión
Amino Acids Zwitterion At ph values above or below the isoelectric. Draw the zwitterion form of a given amino acid. Amino acids are examples of zwitterions. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Thus, amino acids exist in aqueous solution primarily in the form of a dipolar ion, or zwitterion (from the german zwitter, meaning “hybrid”). The charged functional groups connect to the rest of the molecule by covalent bonds. Account for some of the typical properties of amino acids (e.g., high melting points, solubility. At ph values above or below the isoelectric. They are made up of an ammonium or amino group which contains a positive charge. Amino acids are the most common example of zwitterions. Amino acids are therefore soluble crystalline solids. Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids. In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged functional groups. Each amino acid has its own pi value based on the properties of the amino acid. An amino acid molecule can interact within itself to form a zwitterion.
From www.docbrown.info
Structure synthesis amino acids zwitterions acidic basic character Amino Acids Zwitterion Each amino acid has its own pi value based on the properties of the amino acid. Draw the zwitterion form of a given amino acid. Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids. When an amino acid contains both a plus and a minus charge in the backbone, it is called. Amino Acids Zwitterion.
From bioquimicanacabeca.blogspot.com
Aminoácidos (Zwitterions) Amino Acids Zwitterion Thus, amino acids exist in aqueous solution primarily in the form of a dipolar ion, or zwitterion (from the german zwitter, meaning “hybrid”). When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Because of these charges in a zwitterion, there are strong intermolecular. Amino Acids Zwitterion.
From www.youtube.com
Zwitterion Form of Amino Acids YouTube Amino Acids Zwitterion In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged functional groups. They are made up of an ammonium or amino group which contains a positive charge. Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids. Account for some of the typical properties of amino. Amino Acids Zwitterion.
From notesbard.com
Zwitterion Definition, Types, & Examples I NotesBard Amino Acids Zwitterion Account for some of the typical properties of amino acids (e.g., high melting points, solubility. At ph values above or below the isoelectric. In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged functional groups. The charged functional groups connect to the rest of the molecule by covalent bonds. The zwitterion of an. Amino Acids Zwitterion.
From www.youtube.com
The zwitterionic life of amino acids YouTube Amino Acids Zwitterion At ph values above or below the isoelectric. Draw the zwitterion form of a given amino acid. Amino acids are therefore soluble crystalline solids. In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged functional groups. An amino acid molecule can interact within itself to form a zwitterion. The charged functional groups connect. Amino Acids Zwitterion.
From www.slideserve.com
PPT AMINO ACIDS PowerPoint Presentation, free download ID5620728 Amino Acids Zwitterion Account for some of the typical properties of amino acids (e.g., high melting points, solubility. Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids. In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged functional groups. Amino acids are the most common example of zwitterions.. Amino Acids Zwitterion.
From www.shutterstock.com
C4h7no4 Zwitterion Aspartic Acid Amino Acid Stock Vector (Royalty Free Amino Acids Zwitterion Amino acids are therefore soluble crystalline solids. The charged functional groups connect to the rest of the molecule by covalent bonds. Account for some of the typical properties of amino acids (e.g., high melting points, solubility. Each amino acid has its own pi value based on the properties of the amino acid. Thus, amino acids exist in aqueous solution primarily. Amino Acids Zwitterion.
From www.vectorstock.com
C3h7no3 zwitterion of serine amino acid molecule Vector Image Amino Acids Zwitterion In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged functional groups. Draw the zwitterion form of a given amino acid. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. They are made up of an ammonium or amino group which contains a positive charge. Each amino. Amino Acids Zwitterion.
From es-academic.com
Zwitterión Amino Acids Zwitterion The zwitterion of an amino acid exists at a ph equal to the isoelectric point. They are made up of an ammonium or amino group which contains a positive charge. An amino acid molecule can interact within itself to form a zwitterion. When an amino acid contains both a plus and a minus charge in the backbone, it is called. Amino Acids Zwitterion.
From chemistryguru.com.sg
Deduce Zwitterion and Isoelectric Point of Amino Acids Amino Acids Zwitterion At ph values above or below the isoelectric. Amino acids are the most common example of zwitterions. They are made up of an ammonium or amino group which contains a positive charge. Amino acids are examples of zwitterions. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. The charged functional groups connect to the. Amino Acids Zwitterion.
From sciencenotes.org
Zwitterion Definition and Examples Amino Acids Zwitterion An amino acid molecule can interact within itself to form a zwitterion. At ph values above or below the isoelectric. Thus, amino acids exist in aqueous solution primarily in the form of a dipolar ion, or zwitterion (from the german zwitter, meaning “hybrid”). Amino acids are therefore soluble crystalline solids. Draw the zwitterion form of a given amino acid. Amino. Amino Acids Zwitterion.
From www.chemistrystudent.com
Amino Acids and Proteins (ALevel) ChemistryStudent Amino Acids Zwitterion An amino acid molecule can interact within itself to form a zwitterion. Draw the zwitterion form of a given amino acid. In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged functional groups. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Amino acids are the most. Amino Acids Zwitterion.
From www.vectorstock.com
C3H7NO2 zwitterion of alanine amino acid molecule Vector Image Amino Acids Zwitterion At ph values above or below the isoelectric. Amino acids are the most common example of zwitterions. In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged functional groups. The charged functional groups connect to the rest of the molecule by covalent bonds. Draw the zwitterion form of a given amino acid. Because. Amino Acids Zwitterion.
From www3.nd.edu
Zwitterionic form of the aamino acids that occur vat physiological pH Amino Acids Zwitterion The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Each amino acid has its own pi value based on the properties of the amino acid. Amino acids are examples of zwitterions. Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids. When an amino acid contains both. Amino Acids Zwitterion.
From byjus.com
An amino acid under certain conditions has both positive and negative Amino Acids Zwitterion Amino acids are examples of zwitterions. The charged functional groups connect to the rest of the molecule by covalent bonds. In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged functional groups. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Thus, amino acids exist in aqueous. Amino Acids Zwitterion.
From quizlet.com
What is a zwitterion? Under what conditions do amino acids b Quizlet Amino Acids Zwitterion Draw the zwitterion form of a given amino acid. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Thus, amino acids exist in aqueous solution primarily in the form of a dipolar ion, or zwitterion (from the german zwitter, meaning “hybrid”). Each amino. Amino Acids Zwitterion.
From www.simplemed.co.uk
3. Structure of Proteins SimpleMed Learning Medicine, Simplified Amino Acids Zwitterion Amino acids are the most common example of zwitterions. They are made up of an ammonium or amino group which contains a positive charge. Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids. Thus, amino acids exist in aqueous solution primarily in the form of a dipolar ion, or zwitterion (from the. Amino Acids Zwitterion.
From conductscience.com
Amino Acids Building Blocks of Proteins Conduct Science Amino Acids Zwitterion Thus, amino acids exist in aqueous solution primarily in the form of a dipolar ion, or zwitterion (from the german zwitter, meaning “hybrid”). An amino acid molecule can interact within itself to form a zwitterion. They are made up of an ammonium or amino group which contains a positive charge. Because of these charges in a zwitterion, there are strong. Amino Acids Zwitterion.
From www.alamy.com
General formula of amino acids, ionized and nonionized (zwitterion Amino Acids Zwitterion Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids. The charged functional groups connect to the rest of the molecule by covalent bonds. Amino acids are examples of zwitterions. At ph values above or below the isoelectric. In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and. Amino Acids Zwitterion.
From stock.adobe.com
Vetor de Chemical structure of amino acid or zwitterion do Stock Amino Acids Zwitterion When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Amino acids are the most common example of zwitterions. Amino acids are examples of zwitterions. The charged functional groups connect to the rest of the molecule by covalent bonds. Because of these charges in. Amino Acids Zwitterion.
From glossary.periodni.com
Zwitterion Chemistry Dictionary & Glossary Amino Acids Zwitterion Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids. The charged functional groups connect to the rest of the molecule by covalent bonds. They are made up of an ammonium or amino group which contains a positive charge. Thus, amino acids exist in aqueous solution primarily in the form of a dipolar. Amino Acids Zwitterion.
From leah4sci.com
Zwitterion and Amino Acid Charge MCAT Biochemistry Tutorial Video Amino Acids Zwitterion When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Amino acids. Amino Acids Zwitterion.
From www.youtube.com
General structure of amino acid; Zwitterion ; Isoelectric Point YouTube Amino Acids Zwitterion At ph values above or below the isoelectric. Amino acids are examples of zwitterions. An amino acid molecule can interact within itself to form a zwitterion. Each amino acid has its own pi value based on the properties of the amino acid. Amino acids are therefore soluble crystalline solids. Amino acids are the most common example of zwitterions. Draw the. Amino Acids Zwitterion.
From trangtraigarung.com
What Is The Zwitterionic Form Of An Amino Acid? Amino Acids Zwitterion An amino acid molecule can interact within itself to form a zwitterion. Account for some of the typical properties of amino acids (e.g., high melting points, solubility. Each amino acid has its own pi value based on the properties of the amino acid. Thus, amino acids exist in aqueous solution primarily in the form of a dipolar ion, or zwitterion. Amino Acids Zwitterion.
From www.pinterest.com
Zwitterion state?! Review Charged Amino Acids brush up on amino acids Amino Acids Zwitterion Each amino acid has its own pi value based on the properties of the amino acid. An amino acid molecule can interact within itself to form a zwitterion. In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged functional groups. Amino acids are therefore soluble crystalline solids. Thus, amino acids exist in aqueous. Amino Acids Zwitterion.
From www.dreamstime.com
C3H7NO2S Zwitterion of Cysteine Amino Acid Molecule Stock Vector Amino Acids Zwitterion At ph values above or below the isoelectric. An amino acid molecule can interact within itself to form a zwitterion. Amino acids are examples of zwitterions. Amino acids are therefore soluble crystalline solids. Each amino acid has its own pi value based on the properties of the amino acid. Draw the zwitterion form of a given amino acid. Because of. Amino Acids Zwitterion.
From www.lecturio.com
Amino Acids & Proteins Chemistry for Physicians Medical Library Amino Acids Zwitterion An amino acid molecule can interact within itself to form a zwitterion. Amino acids are therefore soluble crystalline solids. In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged functional groups. Each amino acid has its own pi value based on the properties of the amino acid. At ph values above or below. Amino Acids Zwitterion.
From chemistryguru.com.sg
Deduce Zwitterion and Isoelectric Point of Amino Acids Amino Acids Zwitterion At ph values above or below the isoelectric. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Amino acids are examples of zwitterions. Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids. They are made up of an ammonium or amino group which contains a positive. Amino Acids Zwitterion.
From www.aquaportail.com
Zwitterion définition et explications Amino Acids Zwitterion Amino acids are examples of zwitterions. Draw the zwitterion form of a given amino acid. They are made up of an ammonium or amino group which contains a positive charge. At ph values above or below the isoelectric. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Each amino acid has its own pi. Amino Acids Zwitterion.
From www.expii.com
Zwitterions — Definition & Importance Expii Amino Acids Zwitterion An amino acid molecule can interact within itself to form a zwitterion. Amino acids are therefore soluble crystalline solids. Account for some of the typical properties of amino acids (e.g., high melting points, solubility. At ph values above or below the isoelectric. Amino acids are the most common example of zwitterions. Because of these charges in a zwitterion, there are. Amino Acids Zwitterion.
From www.vectorstock.com
C2h5no2 zwitterion glycine amino acid molecule Vector Image Amino Acids Zwitterion The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Account for some of the typical properties of amino acids (e.g., high melting points, solubility. Draw the zwitterion form of a given amino acid. Amino acids are therefore soluble crystalline solids. They are made up of an ammonium or amino group which contains a positive. Amino Acids Zwitterion.
From www.vectorstock.com
C4H7NO4 zwitterion of aspartic acid amino acid Vector Image Amino Acids Zwitterion Thus, amino acids exist in aqueous solution primarily in the form of a dipolar ion, or zwitterion (from the german zwitter, meaning “hybrid”). When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Amino acids are the most common example of zwitterions. An amino. Amino Acids Zwitterion.
From www.science-revision.co.uk
Amino acids Amino Acids Zwitterion The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Draw the zwitterion form of a given amino acid. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. In chemistry, a zwitterion is a neutral molecule that has. Amino Acids Zwitterion.
From integrated-mcat.com
Amino Acid In Zwitterion Form Integrated MCAT Course Amino Acids Zwitterion They are made up of an ammonium or amino group which contains a positive charge. Amino acids are examples of zwitterions. Amino acids are therefore soluble crystalline solids. Account for some of the typical properties of amino acids (e.g., high melting points, solubility. In chemistry, a zwitterion is a neutral molecule that has equal numbers of positive and negative charged. Amino Acids Zwitterion.
From www.answersarena.com
[Solved] 4. Draw the zwitterion structure for each of the Amino Acids Zwitterion An amino acid molecule can interact within itself to form a zwitterion. The charged functional groups connect to the rest of the molecule by covalent bonds. Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids. When an amino acid contains both a plus and a minus charge in the backbone, it is. Amino Acids Zwitterion.