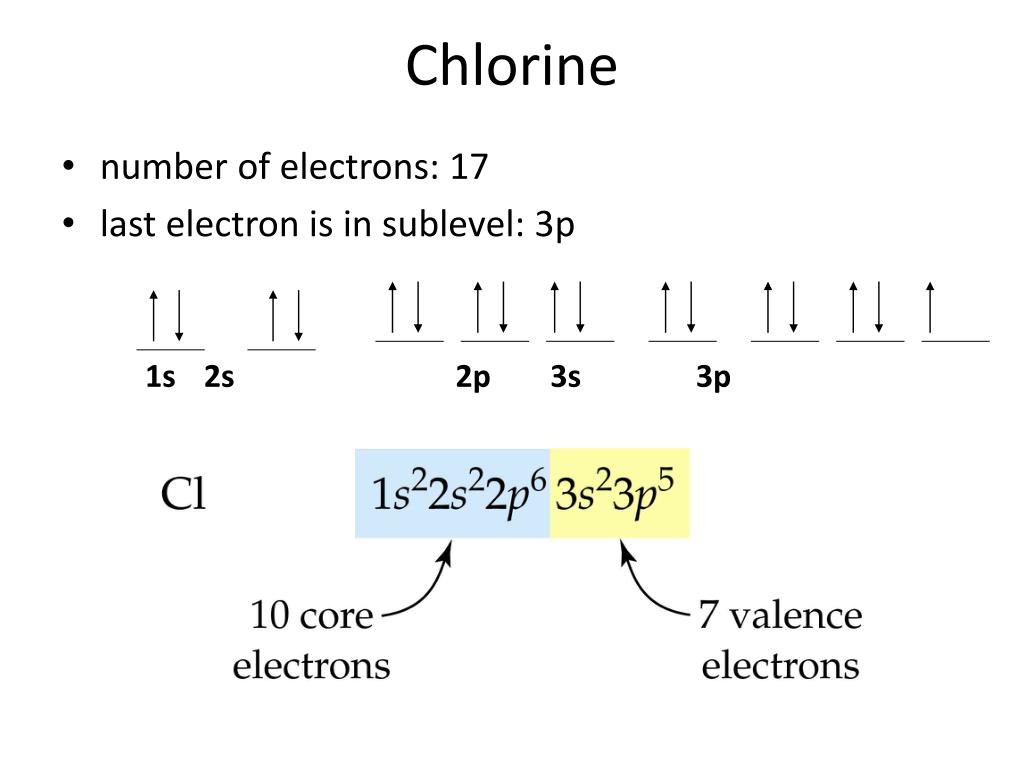
Chlorine Electron Configuration Excited State . 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: 1s 2 2s 2 2p 6 3s 2 3p 5. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). For example, the ground state electronic configuration of chlorine is. 2) the electron configuration of chlorine is: Add an electron to the anion electron configuration. [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. Thus, the electron configuration of. Chlorine has the atomic number 17. Its 17 electrons are distributed as follows: It helps explain the element’s reactivity and. Understanding the electron configuration of chlorine is essential for chemistry enthusiasts. Full ground state electron configuration: 3) what is the molecular geometry of the following? 2 (in k shell), 8 (in l shell), and 7 (in m shell).
from mavink.com
Chlorine has the atomic number 17. Understanding the electron configuration of chlorine is essential for chemistry enthusiasts. Full ground state electron configuration: 2) the electron configuration of chlorine is: [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: 1s 2 2s 2 2p 6 3s 2 3p 5. It helps explain the element’s reactivity and. For example, the ground state electronic configuration of chlorine is. Thus, the electron configuration of.
Orbital Diagram Of Chlorine
Chlorine Electron Configuration Excited State 3) what is the molecular geometry of the following? In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). 1s 2 2s 2 2p 6 3s 2 3p 5. Thus, the electron configuration of. Understanding the electron configuration of chlorine is essential for chemistry enthusiasts. Add an electron to the anion electron configuration. 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: Its 17 electrons are distributed as follows: 2 (in k shell), 8 (in l shell), and 7 (in m shell). Chlorine has the atomic number 17. 2) the electron configuration of chlorine is: 3) what is the molecular geometry of the following? Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. It helps explain the element’s reactivity and. [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. For example, the ground state electronic configuration of chlorine is.
From www.toppr.com
Chlorine atom in its third excited state with fluorine to form a Chlorine Electron Configuration Excited State Understanding the electron configuration of chlorine is essential for chemistry enthusiasts. 2) the electron configuration of chlorine is: Chlorine has the atomic number 17. It helps explain the element’s reactivity and. For example, the ground state electronic configuration of chlorine is. Add an electron to the anion electron configuration. 3) what is the molecular geometry of the following? Thus, the. Chlorine Electron Configuration Excited State.
From www.numerade.com
SOLVED What is covalency of chlorine atom in second excited state Chlorine Electron Configuration Excited State Full ground state electron configuration: For example, the ground state electronic configuration of chlorine is. 2 (in k shell), 8 (in l shell), and 7 (in m shell). It helps explain the element’s reactivity and. 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: 1s 2 2s 2 2p 6 3s 2 3p 5. Understanding the electron configuration of chlorine is essential for. Chlorine Electron Configuration Excited State.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Electron Configuration Excited State Chlorine has the atomic number 17. Add an electron to the anion electron configuration. Full ground state electron configuration: Thus, the electron configuration of. It helps explain the element’s reactivity and. For example, the ground state electronic configuration of chlorine is. 2) the electron configuration of chlorine is: 2 (in k shell), 8 (in l shell), and 7 (in m. Chlorine Electron Configuration Excited State.
From www.dreamstime.com
Electron of the Element Chlorine Stock Vector Illustration of Chlorine Electron Configuration Excited State For example, the ground state electronic configuration of chlorine is. 1s 2 2s 2 2p 6 3s 2 3p 5. 2 (in k shell), 8 (in l shell), and 7 (in m shell). Add an electron to the anion electron configuration. Thus, the electron configuration of. Full ground state electron configuration: Commonly, the electron configuration is used to describe the. Chlorine Electron Configuration Excited State.
From enginelistchester.z5.web.core.windows.net
Chlorine Electron Dot Diagram Chlorine Electron Configuration Excited State 3) what is the molecular geometry of the following? 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: For example, the ground state electronic configuration of chlorine is. 2 (in k shell), 8 (in l shell), and 7 (in m shell). It helps explain the element’s reactivity and. 1s 2 2s 2 2p 6 3s 2 3p 5. Commonly, the electron configuration is. Chlorine Electron Configuration Excited State.
From www.youtube.com
Chlorine Ground State Electron Configuration YouTube Chlorine Electron Configuration Excited State In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Add an electron to the anion electron configuration. It helps explain the element’s reactivity and. 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: 3) what is the molecular geometry of the following? Thus, the electron configuration of.. Chlorine Electron Configuration Excited State.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements Chlorine Electron Configuration Excited State Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. 3) what is the molecular geometry of the following? Full ground state electron configuration: For example, the ground state electronic configuration of chlorine is. It helps. Chlorine Electron Configuration Excited State.
From pixels.com
Chlorine Electron Configuration Photograph by Chlorine Electron Configuration Excited State Add an electron to the anion electron configuration. Full ground state electron configuration: 2 (in k shell), 8 (in l shell), and 7 (in m shell). 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: Thus, the electron configuration of. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are. Chlorine Electron Configuration Excited State.
From stock.adobe.com
Chlorine atomic structure has atomic number, atomic mass, electron Chlorine Electron Configuration Excited State 3) what is the molecular geometry of the following? 1s 2 2s 2 2p 6 3s 2 3p 5. [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to. Chlorine Electron Configuration Excited State.
From www.slideserve.com
PPT The University of the State of New York REGENTS HIGH SCHOOL Chlorine Electron Configuration Excited State Its 17 electrons are distributed as follows: Understanding the electron configuration of chlorine is essential for chemistry enthusiasts. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. In order to write the chlorine electron configuration. Chlorine Electron Configuration Excited State.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Chlorine Electron Configuration Excited State [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. 1s 2 2s 2 2p 6 3s 2 3p 5. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: Its 17. Chlorine Electron Configuration Excited State.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Electron Configuration Excited State For example, the ground state electronic configuration of chlorine is. Add an electron to the anion electron configuration. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Full ground state electron configuration: It helps explain the element’s reactivity and. 2) the electron configuration of. Chlorine Electron Configuration Excited State.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Chlorine Electron Configuration Excited State It helps explain the element’s reactivity and. For example, the ground state electronic configuration of chlorine is. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: 3) what is the molecular geometry of the following? [ne] 3s^2 3p^5. Chlorine Electron Configuration Excited State.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Electron Configuration Excited State Thus, the electron configuration of. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. It helps explain the element’s reactivity and. 2 (in k shell), 8 (in l shell), and 7 (in m shell). Its. Chlorine Electron Configuration Excited State.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Electron Configuration Excited State Add an electron to the anion electron configuration. Thus, the electron configuration of. 2) the electron configuration of chlorine is: Chlorine has the atomic number 17. Its 17 electrons are distributed as follows: [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. Full ground state electron configuration: Understanding the electron configuration of. Chlorine Electron Configuration Excited State.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Electron Configuration Excited State Chlorine has the atomic number 17. It helps explain the element’s reactivity and. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). 1s 2 2s 2 2p 6 3s 2 3p 5. Its 17 electrons are distributed as follows: For example, the ground state. Chlorine Electron Configuration Excited State.
From saylordotorg.github.io
Ions Chlorine Electron Configuration Excited State 2 (in k shell), 8 (in l shell), and 7 (in m shell). Add an electron to the anion electron configuration. 3) what is the molecular geometry of the following? Full ground state electron configuration: It helps explain the element’s reactivity and. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but. Chlorine Electron Configuration Excited State.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Electron Configuration Excited State 1s 2 2s 2 2p 6 3s 2 3p 5. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. 3) what is the molecular geometry of the following? For example, the ground state electronic configuration. Chlorine Electron Configuration Excited State.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine Electron Configuration Excited State It helps explain the element’s reactivity and. 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Understanding the electron configuration of chlorine is essential for chemistry enthusiasts. Thus, the electron configuration of. 2) the electron configuration of chlorine. Chlorine Electron Configuration Excited State.
From www.slideshare.net
Atomic concepts Chlorine Electron Configuration Excited State Add an electron to the anion electron configuration. 2 (in k shell), 8 (in l shell), and 7 (in m shell). Chlorine has the atomic number 17. [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. Its 17 electrons are distributed as follows: Full ground state electron configuration: 1s 2 2s 2. Chlorine Electron Configuration Excited State.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Electron Configuration Excited State 3) what is the molecular geometry of the following? 2 (in k shell), 8 (in l shell), and 7 (in m shell). [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. Chlorine has the atomic number 17. It helps explain the element’s reactivity and. In order to write the chlorine electron configuration. Chlorine Electron Configuration Excited State.
From ar.inspiredpencil.com
Orbital Diagram For Chlorine Chlorine Electron Configuration Excited State 2 (in k shell), 8 (in l shell), and 7 (in m shell). It helps explain the element’s reactivity and. Add an electron to the anion electron configuration. 1s 2 2s 2 2p 6 3s 2 3p 5. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. Chlorine Electron Configuration Excited State.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Electron Configuration Excited State Understanding the electron configuration of chlorine is essential for chemistry enthusiasts. 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: It helps explain the element’s reactivity and. 3) what is the molecular geometry of the following? [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. 2) the electron configuration of chlorine is: 1s 2 2s. Chlorine Electron Configuration Excited State.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Electron Configuration Excited State Add an electron to the anion electron configuration. It helps explain the element’s reactivity and. Full ground state electron configuration: For example, the ground state electronic configuration of chlorine is. 3) what is the molecular geometry of the following? Understanding the electron configuration of chlorine is essential for chemistry enthusiasts. [ne] 3s^2 3p^5 chlorine has an atomic number of 17,. Chlorine Electron Configuration Excited State.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Electron Configuration Excited State Thus, the electron configuration of. 2 (in k shell), 8 (in l shell), and 7 (in m shell). Add an electron to the anion electron configuration. Chlorine has the atomic number 17. Full ground state electron configuration: 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state,. Chlorine Electron Configuration Excited State.
From www.webelements.com
Elements Periodic Table » Chlorine » properties of free atoms Chlorine Electron Configuration Excited State Understanding the electron configuration of chlorine is essential for chemistry enthusiasts. Full ground state electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 5. It helps explain the element’s reactivity and. Thus, the electron configuration of. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be. Chlorine Electron Configuration Excited State.
From www.coursehero.com
[Solved] Identify the neutral element represented by this excited‑state Chlorine Electron Configuration Excited State 2 (in k shell), 8 (in l shell), and 7 (in m shell). In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. Thus, the electron configuration of.. Chlorine Electron Configuration Excited State.
From valenceelectrons.com
How to Write the Electron Configuration for Chlorine (Cl)? Chlorine Electron Configuration Excited State Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. For example, the ground state electronic configuration of chlorine. Chlorine Electron Configuration Excited State.
From sciencenotes.org
Chlorine Facts Chlorine Electron Configuration Excited State Add an electron to the anion electron configuration. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Understanding the electron configuration of chlorine is essential for chemistry enthusiasts. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground. Chlorine Electron Configuration Excited State.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Electron Configuration Excited State 2 (in k shell), 8 (in l shell), and 7 (in m shell). [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: Understanding the electron configuration of chlorine is essential for chemistry enthusiasts. Add an electron to the anion electron configuration. For example, the ground state. Chlorine Electron Configuration Excited State.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine Electron Configuration Excited State [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. 1s 2 2s 2 2p 6 3s 2 3p. Chlorine Electron Configuration Excited State.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chlorine Electron Configuration Excited State [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. It helps explain the element’s reactivity and. 2) the electron configuration of chlorine is: 1s 2 2s 2 2p 6 3s 2 3p 5. 2 (in k shell), 8 (in l shell), and 7 (in m shell). Its 17 electrons are distributed as. Chlorine Electron Configuration Excited State.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Chlorine Electron Configuration Excited State Understanding the electron configuration of chlorine is essential for chemistry enthusiasts. It helps explain the element’s reactivity and. 1s^2 2s^2 2p^6 3s^2 3p^5 abbreviated: 2) the electron configuration of chlorine is: Chlorine has the atomic number 17. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used. Chlorine Electron Configuration Excited State.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Electron Configuration Excited State For example, the ground state electronic configuration of chlorine is. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). 2) the electron configuration of chlorine is: Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but. Chlorine Electron Configuration Excited State.
From mavink.com
Orbital Diagram Of Chlorine Chlorine Electron Configuration Excited State Thus, the electron configuration of. [ne] 3s^2 3p^5 chlorine has an atomic number of 17, which means it has 17 protons and. 3) what is the molecular geometry of the following? It helps explain the element’s reactivity and. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be. Chlorine Electron Configuration Excited State.