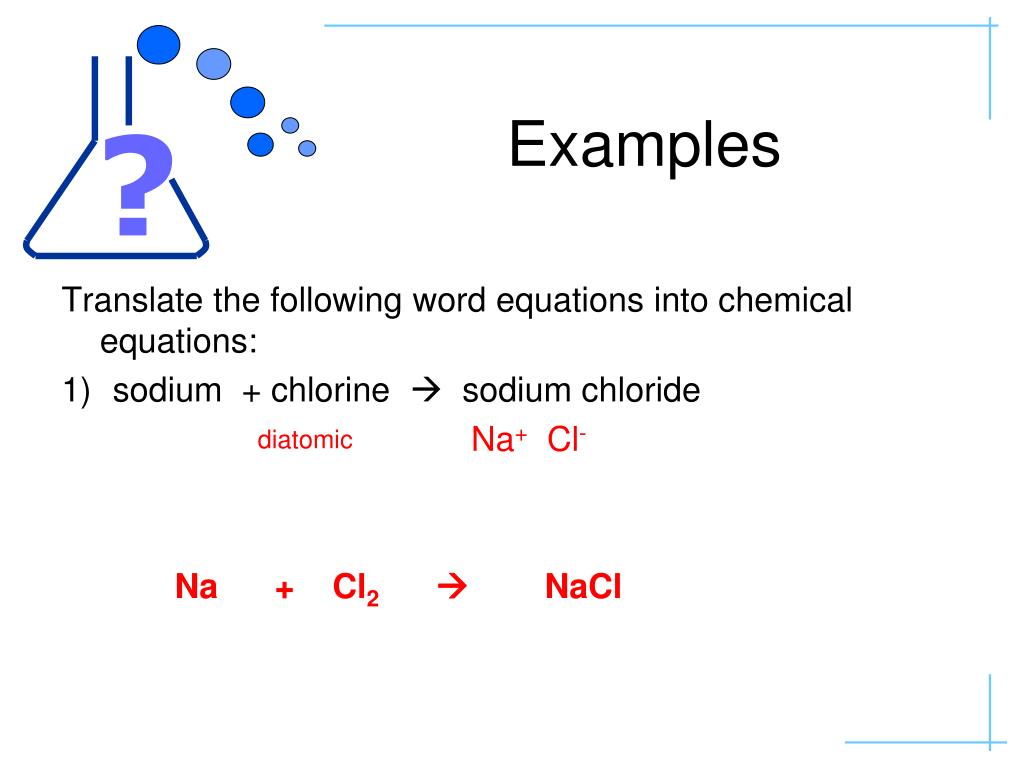
Chlorine Word Equation . “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in. Cl + ki = kcl + i2 is a single displacement (substitution) reaction. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. The chemicals produced were a solution of calcium chloride,. Chlorine + potassium iodide = potassium chloride + diiodine. Chlorine + potassium bromide → bromine + potassium chloride: Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. Convert word equations into chemical equations. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation.
from www.slideserve.com
In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. Cl + ki = kcl + i2 is a single displacement (substitution) reaction. Convert word equations into chemical equations. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Chlorine + potassium iodide = potassium chloride + diiodine. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation. Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. The chemicals produced were a solution of calcium chloride,.
PPT Chemical Reactions PowerPoint Presentation, free download ID
Chlorine Word Equation Convert word equations into chemical equations. Chlorine + potassium iodide = potassium chloride + diiodine. “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. Chlorine + potassium bromide → bromine + potassium chloride: Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) The chemicals produced were a solution of calcium chloride,. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation. Cl + ki = kcl + i2 is a single displacement (substitution) reaction. Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Convert word equations into chemical equations. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in. In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas.
From www.youtube.com
How to Write the Formula for Chlorine dioxide YouTube Chlorine Word Equation Chlorine + potassium iodide = potassium chloride + diiodine. In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in. Cl + ki = kcl + i2 is a single displacement. Chlorine Word Equation.
From slideplayer.com
Chemical Reactions. ppt download Chlorine Word Equation “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to. Chlorine Word Equation.
From www.youtube.com
Equation for LiCl + H2O (Lithium chloride + Water) YouTube Chlorine Word Equation In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in. Convert word equations into chemical equations. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation. In. Chlorine Word Equation.
From www.slideserve.com
PPT Chemical Reactions Unit PowerPoint Presentation, free download Chlorine Word Equation Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Convert word equations into chemical equations. Chlorine + potassium iodide = potassium chloride + diiodine. Cl + ki = kcl + i2 is a single displacement (substitution) reaction. Chlorine + potassium bromide → bromine + potassium chloride: A word equation should state. Chlorine Word Equation.
From asideload7.gitlab.io
Formidable Word Equation Of Sodium Chloride Edexcel Physics Syllabus Chlorine Word Equation Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. The chemicals produced were a solution of calcium chloride,. Chlorine + potassium iodide = potassium chloride + diiodine. Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. Convert word equations into chemical equations. A word. Chlorine Word Equation.
From www.slideshare.net
Chemical Reactions Chlorine Word Equation Convert word equations into chemical equations. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in. “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. Cl 2 (g). Chlorine Word Equation.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Chlorine Word Equation In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation. Use the common symbols,. Chlorine Word Equation.
From www.slideserve.com
PPT How do you know how many atoms and how many elements are in a Chlorine Word Equation The chemicals produced were a solution of calcium chloride,. Chlorine + potassium iodide = potassium chloride + diiodine. Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) In this equation, there are two sodiums in. Chlorine Word Equation.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Hydrogen Chloride Word Equation Chlorine Word Equation In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. Cl + ki = kcl + i2 is a single displacement (substitution) reaction. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Chlorine + potassium bromide → bromine + potassium chloride: Cl 2 (g) +. Chlorine Word Equation.
From slidetodoc.com
Chemical Reactions Counting Atoms and Balancing Chemical Equations Chlorine Word Equation Chlorine + potassium iodide = potassium chloride + diiodine. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) Chlorine + potassium bromide → bromine + potassium chloride: Use the common symbols, \(\left( s \right)\),. Chlorine Word Equation.
From signalticket9.pythonanywhere.com
First Class Word Equation For Sodium And Chlorine Thermal Energy Chlorine Word Equation Chlorine + potassium bromide → bromine + potassium chloride: In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. Chlorine + potassium iodide = potassium chloride + diiodine. Cl + ki = kcl + i2 is a single displacement (substitution) reaction. Convert word equations into chemical equations. The chemicals produced were a solution of. Chlorine Word Equation.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Chlorine Word Equation The chemicals produced were a solution of calcium chloride,. Convert word equations into chemical equations. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. A word equation should. Chlorine Word Equation.
From slideplayer.com
Chapter 10 Chemical Reactions ppt download Chlorine Word Equation “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. Chlorine + potassium iodide = potassium chloride + diiodine. Cl + ki = kcl + i2 is a single displacement (substitution) reaction. In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq). Chlorine Word Equation.
From slideplayer.com
Chemical Equations Chapter ppt download Chlorine Word Equation “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. Cl + ki = kcl + i2 is a single displacement (substitution) reaction. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation. Convert word equations into chemical equations. In. Chlorine Word Equation.
From www.scribd.com
Balancing Chemical Equations Chloride Chlorine Chlorine Word Equation In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) The chemicals produced were a solution of calcium chloride,. Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. Chlorine + potassium bromide. Chlorine Word Equation.
From slidetodoc.com
Chemical Equations Test Review 2 Label the parts Chlorine Word Equation “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. Chlorine + potassium iodide = potassium chloride + diiodine. Chlorine + potassium bromide → bromine + potassium chloride: The chemicals produced were a solution of calcium chloride,. Diatomic chlorine and sodium hydroxide (lye). Chlorine Word Equation.
From studylib.net
Write word equations for the following reactions. Include reactants Chlorine Word Equation The chemicals produced were a solution of calcium chloride,. Cl + ki = kcl + i2 is a single displacement (substitution) reaction. Chlorine + potassium bromide → bromine + potassium chloride: In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. A word equation should state the reactants (starting materials), products (ending materials), and. Chlorine Word Equation.
From signalticket9.pythonanywhere.com
First Class Word Equation For Sodium And Chlorine Thermal Energy Chlorine Word Equation In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation. Convert word equations into chemical equations. In this equation, there are two sodiums in the. Chlorine Word Equation.
From slideplayer.com
Chapter 4 Chemical Reactions ppt download Chlorine Word Equation Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation. Cl + ki = kcl + i2 is. Chlorine Word Equation.
From www.numerade.com
SOLVED The formation of sodium chloride from its elements is given by Chlorine Word Equation Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. Chlorine + potassium iodide = potassium chloride + diiodine. Convert word equations into chemical equations. “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) A word equation should state the reactants (starting materials), products (ending. Chlorine Word Equation.
From slideplayer.com
Chemical Equations & Reactions ppt download Chlorine Word Equation Chlorine + potassium bromide → bromine + potassium chloride: A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation. In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. Use the common symbols, \(\left( s. Chlorine Word Equation.
From www.slideserve.com
PPT Chapter 10 Chemical Reactions PowerPoint Presentation, free Chlorine Word Equation A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation. Chlorine + potassium bromide → bromine + potassium chloride: Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. “calcium carbonate powder reacted. Chlorine Word Equation.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Hydrogen Chloride Word Equation Chlorine Word Equation Chlorine + potassium iodide = potassium chloride + diiodine. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in. Convert word equations into chemical equations. “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. The chemicals produced were a solution of calcium chloride,. Use. Chlorine Word Equation.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Chlorine Word Equation A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation. In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) Cl + ki =. Chlorine Word Equation.
From slideplayer.com
Biology Dr. Altstiel Naples American High School ppt download Chlorine Word Equation Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in. Chlorine + potassium iodide = potassium chloride + diiodine. Chlorine + potassium bromide →. Chlorine Word Equation.
From slideplayer.com
Chemical Reactions. ppt download Chlorine Word Equation In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. The chemicals produced were a solution of calcium chloride,. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) Convert word equations into chemical equations. Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. A word equation should state the. Chlorine Word Equation.
From slideplayer.com
Chemical Equations and other fun with chemistry. ppt download Chlorine Word Equation Cl + ki = kcl + i2 is a single displacement (substitution) reaction. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in. Convert word equations into chemical equations. In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. Chlorine. Chlorine Word Equation.
From www.chegg.com
Solved (From BC Science Connections Text) Classifying Chlorine Word Equation Cl + ki = kcl + i2 is a single displacement (substitution) reaction. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. Convert word equations into chemical equations.. Chlorine Word Equation.
From www.youtube.com
Chlorine and Water AS Chemistry YouTube Chlorine Word Equation Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. Chlorine + potassium iodide = potassium chloride + diiodine. The chemicals produced were a solution of calcium chloride,. In this equation, there are two sodiums in the reactants, two sodiums. Chlorine Word Equation.
From brainly.in
Chlorine reacts with potassium bromide. complete the word equation Chlorine Word Equation The chemicals produced were a solution of calcium chloride,. “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. Convert. Chlorine Word Equation.
From www.youtube.com
Type of Reaction for Na + Cl2 = NaCl (Sodium + Chlorine gas) YouTube Chlorine Word Equation Convert word equations into chemical equations. “calcium carbonate powder reacted with a solution of dilute hydrochloric acid. The chemicals produced were a solution of calcium chloride,. Chlorine + potassium bromide → bromine + potassium chloride: Chlorine + potassium iodide = potassium chloride + diiodine. In this equation, there are two sodiums in the reactants, two sodiums in the products, two. Chlorine Word Equation.
From slideplayer.com
Unit 3 Writing Chemical Reactions ppt download Chlorine Word Equation Chlorine + potassium bromide → bromine + potassium chloride: Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) The chemicals produced were a solution of calcium chloride,. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation. In this. Chlorine Word Equation.
From www.chegg.com
Solved Word Equations Write the word equations below as Chlorine Word Equation The chemicals produced were a solution of calcium chloride,. In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. Cl + ki = kcl + i2 is a single displacement (substitution) reaction. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two. Chlorine Word Equation.
From askfilo.com
chlorine atoms. 2. Explain by writing a word equation. Filo Chlorine Word Equation Chlorine + potassium bromide → bromine + potassium chloride: Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g. Cl + ki = kcl + i2 is a single displacement (substitution) reaction. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Chlorine Word Equation.
From www.essentialchemicalindustry.org
Chlorine Chlorine Word Equation The chemicals produced were a solution of calcium chloride,. In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. Cl 2 (g) + 2kbr(aq) → br 2 (aq) + 2kcl(aq) Convert word equations into chemical equations. Chlorine + potassium bromide → bromine + potassium chloride: A word equation should state the reactants (starting materials),. Chlorine Word Equation.