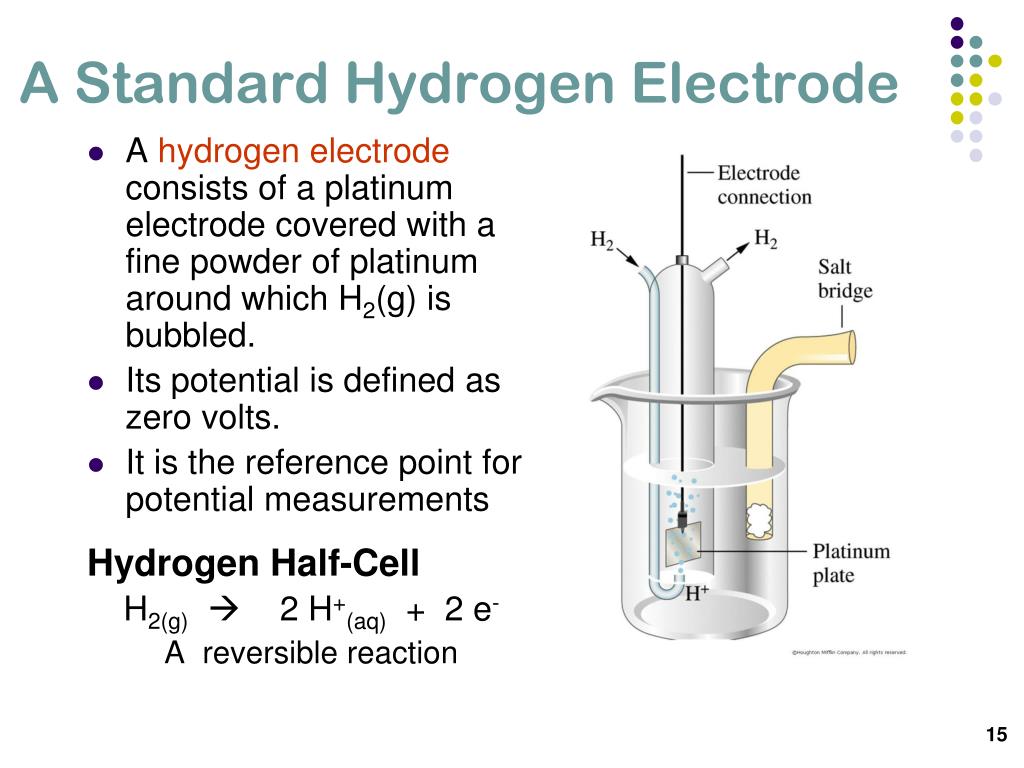
The Standard Hydrogen Electrode Potential Is Zero Because . The standard hydrogen electrode potential is zero because: The potential of a half. Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison with all other electrochemical. We call this \(e^0\), the standard. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. The standard electrode potential of any other electrode is obtained by combining the electrode with she. The value of the standard electrode potential is. B) electrode potential is considered as zero. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k.
from www.slideserve.com
The standard electrode potential of any other electrode is obtained by combining the electrode with she. We call this \(e^0\), the standard. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The value of the standard electrode potential is. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. The potential of a half. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. B) electrode potential is considered as zero. Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison with all other electrochemical.
PPT Electrochemistry PowerPoint Presentation, free download ID5744606
The Standard Hydrogen Electrode Potential Is Zero Because The standard hydrogen electrode potential is zero because: The value of the standard electrode potential is. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. We call this \(e^0\), the standard. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison with all other electrochemical. The standard hydrogen electrode potential is zero because: B) electrode potential is considered as zero. The potential of a half. The standard electrode potential of any other electrode is obtained by combining the electrode with she. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 The Standard Hydrogen Electrode Potential Is Zero Because Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The standard electrode potential of any. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.youtube.com
Standard Reduction Potentials and the Standard Hydrogen Electrode The Standard Hydrogen Electrode Potential Is Zero Because The potential of a half. B) electrode potential is considered as zero. Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison with all other electrochemical. The standard electrode potential of any other electrode is obtained by combining the electrode with she. The potential of the standard hydrogen. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free The Standard Hydrogen Electrode Potential Is Zero Because The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. B) electrode potential is considered as zero. Under these conditions, the potential for the hydrogen reduction is defined. The Standard Hydrogen Electrode Potential Is Zero Because.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled The Standard Hydrogen Electrode Potential Is Zero Because The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. B) electrode potential is considered as zero. The potential of a half. We call this \(e^0\), the standard. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Under these. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.toppr.com
A standard hydrogen electrode has zero electrode potential because The Standard Hydrogen Electrode Potential Is Zero Because The potential of a half. The standard hydrogen electrode potential is zero because: The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. B) electrode potential is considered as zero. The value of the standard electrode potential is. Under these conditions, the potential for the hydrogen. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.toppr.com
The standard hydrogen electrode has zero electrode potential because The Standard Hydrogen Electrode Potential Is Zero Because Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison with all other electrochemical. We call this \(e^0\), the standard. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. B) electrode potential is considered as zero. The potential of a half. The value. The Standard Hydrogen Electrode Potential Is Zero Because.
From issuu.com
standard hydrogen electrode by norainihamzah9 Issuu The Standard Hydrogen Electrode Potential Is Zero Because We call this \(e^0\), the standard. The value of the standard electrode potential is. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The potential of the standard hydrogen electrode (she) is defined as. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.slideserve.com
PPT Cell Potentials at Standard Conditions PowerPoint Presentation The Standard Hydrogen Electrode Potential Is Zero Because The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. B) electrode potential is considered as zero. We call this \(e^0\), the standard. The standard electrode potential of any other electrode is obtained by combining the electrode with she. The value of the standard electrode potential. The Standard Hydrogen Electrode Potential Is Zero Because.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog The Standard Hydrogen Electrode Potential Is Zero Because We call this \(e^0\), the standard. Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison with all other electrochemical. The standard electrode potential of any other electrode is obtained by combining the electrode with she. Since the standard potential of the hydrogen electrode is zero, the measured. The Standard Hydrogen Electrode Potential Is Zero Because.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use The Standard Hydrogen Electrode Potential Is Zero Because Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison with all other electrochemical. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The value of the standard electrode potential is. B) electrode potential is considered as zero. The potential of a half.. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free The Standard Hydrogen Electrode Potential Is Zero Because We call this \(e^0\), the standard. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The standard hydrogen electrode is often abbreviated to she, and its standard. The Standard Hydrogen Electrode Potential Is Zero Because.
From cider.uoregon.edu
Standard Hydrogen Electrode (SHE) Simulation and Animation AACT CIDER The Standard Hydrogen Electrode Potential Is Zero Because Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison with all other electrochemical. The value of the standard electrode potential is. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The standard electrode potential of any other electrode is obtained by combining. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.youtube.com
Class 12 Electrochemistry / How electrode potential is measured by The Standard Hydrogen Electrode Potential Is Zero Because The standard hydrogen electrode potential is zero because: Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison with all other electrochemical. The standard hydrogen electrode is. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube The Standard Hydrogen Electrode Potential Is Zero Because Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be. The Standard Hydrogen Electrode Potential Is Zero Because.
From pandai.me
Standard Electrode Potential The Standard Hydrogen Electrode Potential Is Zero Because We call this \(e^0\), the standard. The value of the standard electrode potential is. The standard hydrogen electrode potential is zero because: The potential of a half. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Under these conditions, the potential for the hydrogen reduction. The Standard Hydrogen Electrode Potential Is Zero Because.
From mungfali.com
Ppt Chapter 17 Electrochemistry Powerpoint Presentation, Free A12 The Standard Hydrogen Electrode Potential Is Zero Because B) electrode potential is considered as zero. Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison with all other electrochemical. We call this \(e^0\), the standard. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The value of the standard. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free The Standard Hydrogen Electrode Potential Is Zero Because Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. We call this \(e^0\), the standard. The standard electrode potential of any other electrode is obtained by combining the electrode with she. The. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.pinterest.com
Standard Hydrogen Electrode Easy Science Reduction potential The Standard Hydrogen Electrode Potential Is Zero Because The standard electrode potential of any other electrode is obtained by combining the electrode with she. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. B) electrode potential is considered as zero. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode.. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.doubtnut.com
A standard hydrogen electrode has zero electrode potential because The Standard Hydrogen Electrode Potential Is Zero Because The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. B) electrode potential is considered as zero. The value of the standard electrode potential is. The potential of the standard hydrogen electrode. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free The Standard Hydrogen Electrode Potential Is Zero Because The standard electrode potential of any other electrode is obtained by combining the electrode with she. B) electrode potential is considered as zero. The standard hydrogen electrode potential is zero because: The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. We call this \(e^0\), the standard. Since the standard potential of the hydrogen. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.slideserve.com
PPT ELECTROCHEMISTRY PowerPoint Presentation, free download ID6102398 The Standard Hydrogen Electrode Potential Is Zero Because The standard hydrogen electrode potential is zero because: The value of the standard electrode potential is. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. Its absolute electrode potential is estimated. The Standard Hydrogen Electrode Potential Is Zero Because.
From byjus.com
What is standard hydrogen electrode? The Standard Hydrogen Electrode Potential Is Zero Because The potential of a half. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The standard electrode potential of any other electrode is obtained by combining the electrode with she. The potential of the. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5744606 The Standard Hydrogen Electrode Potential Is Zero Because Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The value of the standard electrode potential is. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The. The Standard Hydrogen Electrode Potential Is Zero Because.
From chemistnotes.com
Standard hydrogen electrode(SHE) Definition, diagram, application The Standard Hydrogen Electrode Potential Is Zero Because The standard electrode potential of any other electrode is obtained by combining the electrode with she. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. B) electrode potential is considered as zero. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared. The Standard Hydrogen Electrode Potential Is Zero Because.
From www.semanticscholar.org
Figure 3 from Standard hydrogen electrode and potential of zero charge The Standard Hydrogen Electrode Potential Is Zero Because Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison with all other electrochemical. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The standard hydrogen electrode potential is zero because: The standard electrode potential of any other electrode is obtained. The Standard Hydrogen Electrode Potential Is Zero Because.
From saylordotorg.github.io
Standard Potentials The Standard Hydrogen Electrode Potential Is Zero Because B) electrode potential is considered as zero. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The potential of the standard hydrogen electrode (she). The Standard Hydrogen Electrode Potential Is Zero Because.
From www.shutterstock.com
Electrode Potentials Standard Hydrogen Electrode Standard Stock Vector The Standard Hydrogen Electrode Potential Is Zero Because The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The standard hydrogen electrode potential is zero because: Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison. The Standard Hydrogen Electrode Potential Is Zero Because.
From stock.adobe.com
Vetor de A Standard Hydrogen Electrode or SHE is an electrode that The Standard Hydrogen Electrode Potential Is Zero Because Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. We call this \(e^0\), the standard. The potential of a half. The standard hydrogen electrode potential is zero because: The potential of the standard hydrogen. The Standard Hydrogen Electrode Potential Is Zero Because.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation The Standard Hydrogen Electrode Potential Is Zero Because The value of the standard electrode potential is. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The standard electrode potential of any other electrode is obtained by combining the electrode with she. The potential of the standard hydrogen electrode (she) is defined as 0. The Standard Hydrogen Electrode Potential Is Zero Because.
From 2012books.lardbucket.org
Standard Potentials The Standard Hydrogen Electrode Potential Is Zero Because The potential of a half. We call this \(e^0\), the standard. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. Under these conditions, the. The Standard Hydrogen Electrode Potential Is Zero Because.
From sulekharanirxiichemistry.blogspot.com
SULEKHA Class XII Chemistry STANDARD HYDROGEN ELECTRODE The Standard Hydrogen Electrode Potential Is Zero Because The standard electrode potential of any other electrode is obtained by combining the electrode with she. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The potential of a half. The potential of the. The Standard Hydrogen Electrode Potential Is Zero Because.
From monomole.com
Standard hydrogen electrode Mono Mole The Standard Hydrogen Electrode Potential Is Zero Because The potential of a half. Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison with all other electrochemical. The standard electrode potential of any other electrode is obtained by combining the electrode with she. The value of the standard electrode potential is. Under these conditions, the potential. The Standard Hydrogen Electrode Potential Is Zero Because.
From app.pandai.org
Standard Electrode Potential The Standard Hydrogen Electrode Potential Is Zero Because Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. Its absolute electrode potential is estimated to be 4.44 ± 0.02 v at 25 °c, but to form a basis for comparison with all other electrochemical. The potential of a half. B) electrode potential is considered as zero. We. The Standard Hydrogen Electrode Potential Is Zero Because.
From slideplayer.com
Electrochemistry. ppt download The Standard Hydrogen Electrode Potential Is Zero Because B) electrode potential is considered as zero. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The standard hydrogen electrode potential is zero because: Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. Its absolute electrode potential is estimated to be 4.44 ±. The Standard Hydrogen Electrode Potential Is Zero Because.
From app.pandai.org
Standard Electrode Potential The Standard Hydrogen Electrode Potential Is Zero Because The standard electrode potential of any other electrode is obtained by combining the electrode with she. The potential of a half. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. Since the standard potential of the hydrogen electrode is zero, the measured potential is the standard potential of the other electrode. The standard hydrogen electrode. The Standard Hydrogen Electrode Potential Is Zero Because.