Assay Of Tablets . what is a drug assay (test)? the objective is to use one assay method to determine potency of asa and pseh in the same product. An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner.
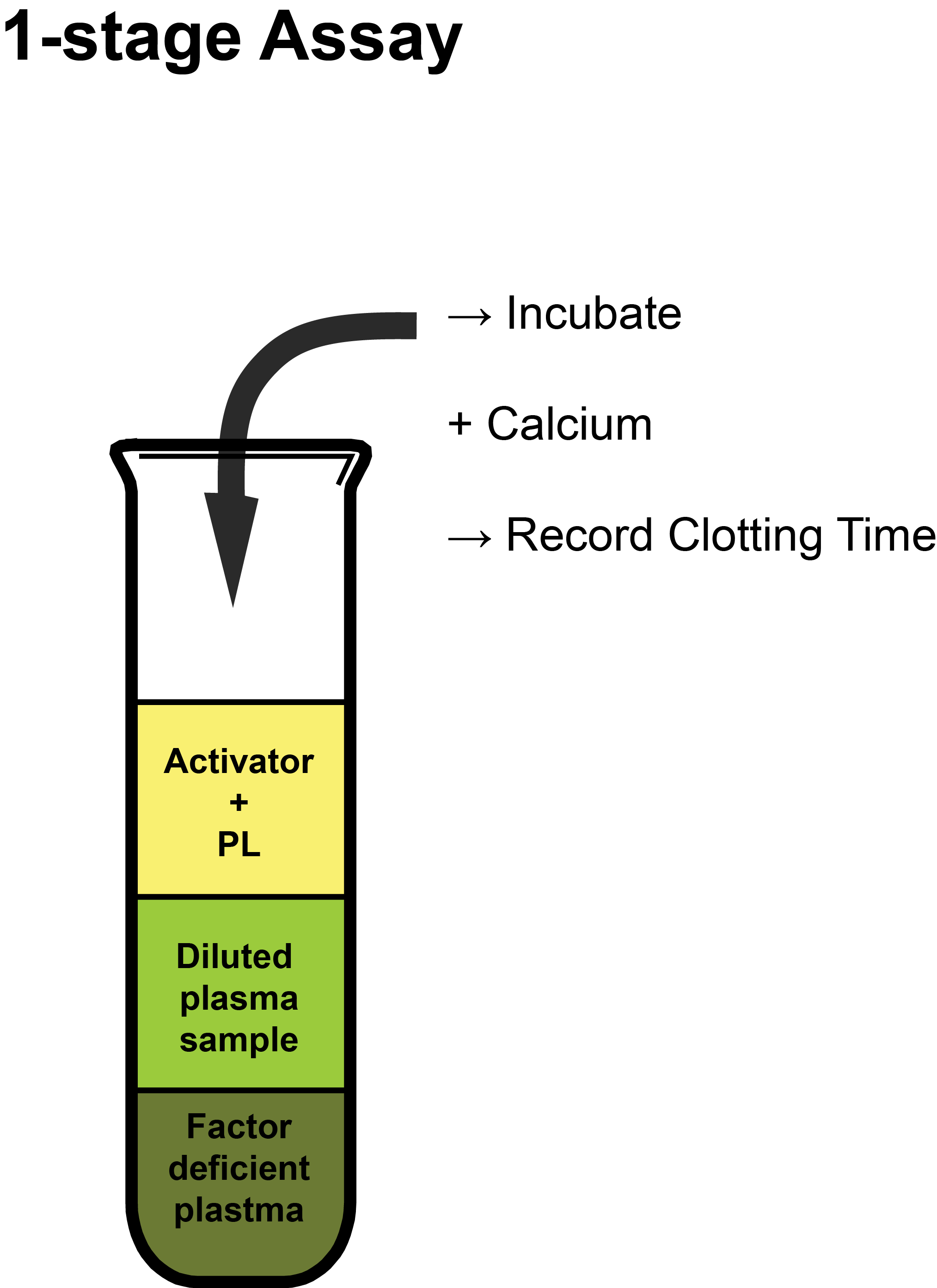
from www.practical-haemostasis.com
a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. what is a drug assay (test)? the objective is to use one assay method to determine potency of asa and pseh in the same product. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets.
1Stage APTTbased Factor Assays
Assay Of Tablets a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. what is a drug assay (test)? a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. the objective is to use one assay method to determine potency of asa and pseh in the same product. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are.
From www.youtube.com
Assay of Aspirin Tablets Dr. Sant Kumar Verma YouTube Assay Of Tablets An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. the objective is to use one assay method to determine potency of asa and pseh in the same product. In a pharmaceutical. Assay Of Tablets.
From pharmacia.pensoft.net
Facile, sensitive and reagentsaving smartphonebased digital image Assay Of Tablets An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. what is a drug assay (test)? a. Assay Of Tablets.
From www.studypool.com
SOLUTION Activity 6 assay of aspirin tablets Studypool Assay Of Tablets In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. in tablet formulation development and during manufacturing of tablet dosage. Assay Of Tablets.
From accroma.com
Application Note Automated Sample Prep for CU and Assay of Assay Of Tablets a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. the objective is to use one assay. Assay Of Tablets.
From www.megazyme.com
Protazyme AK Tablets Protease Activity Assay Megazyme Assay Of Tablets An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. what is a drug assay (test)? a tablet (also known as a pill) is a pharmaceutical oral dosage form. Assay Of Tablets.
From www.researchgate.net
(PDF) Validation of a simple and rapid UV spectrophotometric method for Assay Of Tablets what is a drug assay (test)? An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. in tablet formulation. Assay Of Tablets.
From www.yumpu.com
Validation of Changes to the USP Assay Method for Ibuprofen Tablets Assay Of Tablets in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. the objective is to use one assay method to determine potency of asa and pseh in the same product. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. In a pharmaceutical company,50. Assay Of Tablets.
From www.researchgate.net
(PDF) LC Assay of Eletriptan in Tablets and In Vitro Dissolution Studies Assay Of Tablets in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. what is a drug assay (test)? the objective is to use one assay method to determine potency of asa and pseh in the same product. the method was successfully applied to analyze two marketed tablets in a selective. Assay Of Tablets.
From www.researchgate.net
LBA assay formats for detection of TAb and ADC in the biomatrices. (A Assay Of Tablets An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. what is a. Assay Of Tablets.
From www.researchgate.net
Individual Tablet Assay for Nefersil Tablets (Declared Amount Assay Of Tablets the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. An assay is an investigative or analytic procedure. Assay Of Tablets.
From www.cell.com
MSDbased assays facilitate a rapid and quantitative serostatus Assay Of Tablets In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. what is a drug assay (test)? in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests. Assay Of Tablets.
From www.academia.edu
(PDF) Identification and quantitation assays for intact tablets of two Assay Of Tablets in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. In a pharmaceutical company,50. Assay Of Tablets.
From www.researchgate.net
Tableting assay reagents can achieve increased reagent stability. (a Assay Of Tablets what is a drug assay (test)? In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. the objective is to use one assay method to determine potency of asa and pseh in the. Assay Of Tablets.
From www.youtube.com
lab 7 Assay of Aspirin Tablets YouTube Assay Of Tablets a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. the objective is to use one assay method to determine potency of asa and pseh in the same product. what is a drug assay (test)? In a pharmaceutical company,50 kg api is used to manufacture. Assay Of Tablets.
From slideplayer.com
Experiment three Assay test of ibuprofen tablets ppt download Assay Of Tablets a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. the objective is to use one assay. Assay Of Tablets.
From www.researchgate.net
Precision and accuracy for assay of nimodipine in tablets samples by Assay Of Tablets the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. the objective is. Assay Of Tablets.
From www.sigmachemicals.com.au
Assay Tabs Sigma Chemicals Assay Of Tablets what is a drug assay (test)? In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid. Assay Of Tablets.
From www.youtube.com
Assay of aspirin tablets 2021 YouTube Assay Of Tablets the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. what is a drug assay (test)? An. Assay Of Tablets.
From www.researchgate.net
Impact of assay sensitivity and TAb assay formats on TAb and TI Assay Of Tablets in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. the objective is to use one assay method to determine potency of asa and pseh in the same product. a tablet (also known as a pill) is. Assay Of Tablets.
From www.practical-haemostasis.com
1Stage APTTbased Factor Assays Assay Of Tablets the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. what is a drug assay (test)? An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality. Assay Of Tablets.
From studylib.net
assay of paracetamol tablets Assay Of Tablets In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. the objective is to use one assay method to determine potency of asa and pseh in the same product. An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. the method was successfully applied to analyze. Assay Of Tablets.
From www.researchgate.net
(PDF) FORMULATION DEVELOPMENT AND STABILITY INDICATING HPLC ASSAY OF Assay Of Tablets a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. the objective is to. Assay Of Tablets.
From www.researchgate.net
(PDF) Validation of assay method of amlodipine in tablets by liquid Assay Of Tablets what is a drug assay (test)? the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. . Assay Of Tablets.
From laboratorygroupa6.blogspot.com
TECHNOLOGY PHARMACEUTICAL LABORATORY REPORT GROUP A6 PRACTICAL 7 Assay Of Tablets a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a.. Assay Of Tablets.
From www.studocu.com
Assayofaspirintablets Perform the Assay of aspirin tablets Apparatus Assay Of Tablets In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. what is a drug assay (test)? An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. the. Assay Of Tablets.
From www.researchgate.net
(PDF) Loratadine Tablets' Assay Using an Adjusted USP Method with Assay Of Tablets in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid. Assay Of Tablets.
From www.youtube.com
HPLC Assay Calculation Percentage Purity of Tablet YouTube Assay Of Tablets the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. what is a drug assay (test)? In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. An assay is an investigative. Assay Of Tablets.
From www.pharmacalculation.com
Potency or Assay Calculation in Pharmaceutical Industry Assay Of Tablets the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. what is a drug assay (test)? in tablet formulation. Assay Of Tablets.
From www.youtube.com
WEIGHT VARIATION TEST FOR TABLET DOSAGE FORM EVALUATION PARAMETERS OF Assay Of Tablets An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control. Assay Of Tablets.
From www.researchgate.net
(PDF) HPLC assay of acetylsalicylic acid, paracetamol, caffeine and Assay Of Tablets the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. An assay is an investigative or analytic procedure for assessing or measuring the presence, amount, or functional activity of a. what is a drug assay (test)? in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality. Assay Of Tablets.
From www.researchgate.net
Differences between RMSECs and RMSEVs of assay models (A Aziwok 500 Assay Of Tablets the objective is to use one assay method to determine potency of asa and pseh in the same product. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. a tablet (also. Assay Of Tablets.
From www.semanticscholar.org
Figure 2 from Assay of paracetamol tablets from different manufacturing Assay Of Tablets a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. what is a drug assay (test)? An assay is an investigative or analytic procedure for assessing or. Assay Of Tablets.
From studylib.net
HPLC Assay and Stability Studies of Tablets Containing Assay Of Tablets the objective is to use one assay method to determine potency of asa and pseh in the same product. in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. In a pharmaceutical company,50. Assay Of Tablets.
From pubs.rsc.org
Assay of mebendazole in tablets by highperformance liquid Assay Of Tablets the method was successfully applied to analyze two marketed tablets in a selective and reproducible manner. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. what is a drug assay (test)? in tablet formulation development and during manufacturing of tablet dosage forms, a number of quality control tests are. the objective is to. Assay Of Tablets.
From www.pharmaexcipients.com
A Fast and Nondestructive Terahertz Dissolution Assay for Immediate Assay Of Tablets the objective is to use one assay method to determine potency of asa and pseh in the same product. a tablet (also known as a pill) is a pharmaceutical oral dosage form (oral solid dosage, or osd) or solid unit dosage. In a pharmaceutical company,50 kg api is used to manufacture 100,000 tablets. in tablet formulation development. Assay Of Tablets.