Does Solid Liquid Extraction Used Boiling Point . Usually one of the solvents is water. It purifies a substance only in its liquid phase. Solvent partitioning requires two solvents that are not miscible in each other. The biggest difference between these two processes is this: It purifies a substance whether it is in its liquid or solid phase. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether).
from www.youtube.com
It purifies a substance only in its liquid phase. The biggest difference between these two processes is this: It purifies a substance whether it is in its liquid or solid phase. Solvent partitioning requires two solvents that are not miscible in each other. Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether).
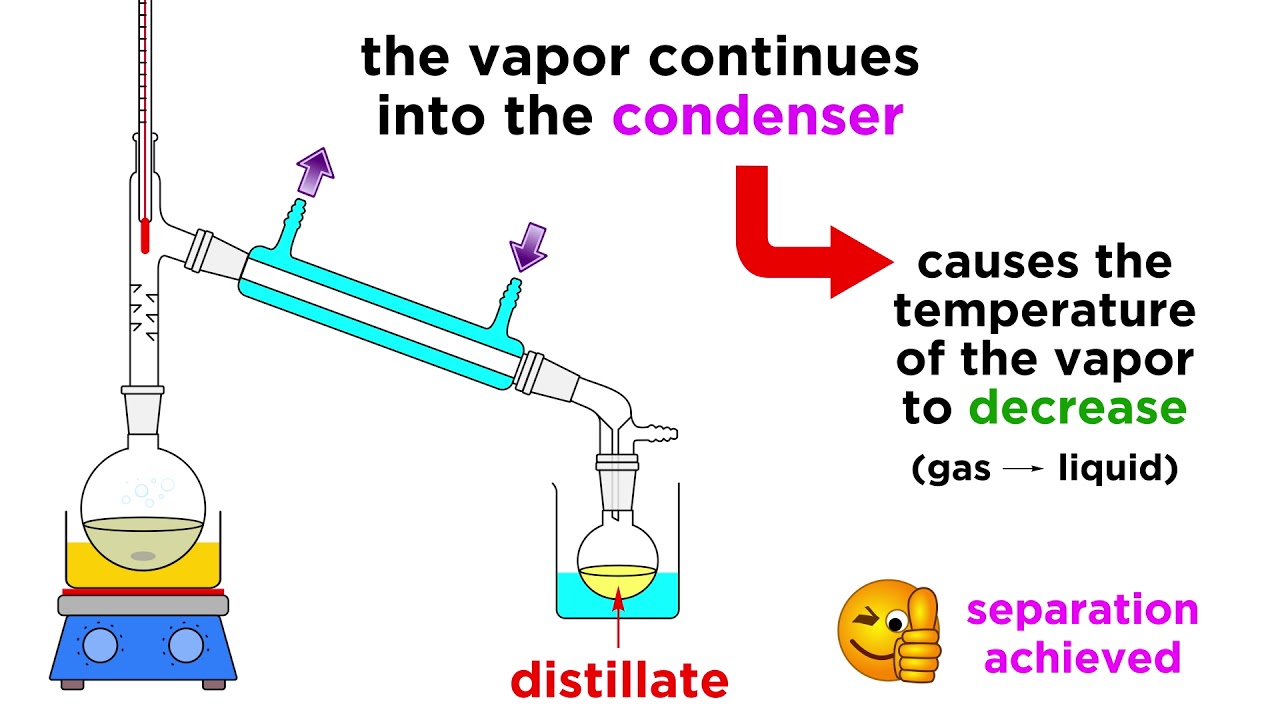
Separating Liquids by Distillation YouTube
Does Solid Liquid Extraction Used Boiling Point Usually one of the solvents is water. It purifies a substance whether it is in its liquid or solid phase. It purifies a substance only in its liquid phase. Solvent partitioning requires two solvents that are not miscible in each other. The biggest difference between these two processes is this: The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). Usually one of the solvents is water.
From www.researchgate.net
Diagrammatic illustration of liquidliquid extraction (adapted from Does Solid Liquid Extraction Used Boiling Point The biggest difference between these two processes is this: The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). Usually one of the solvents is water. It purifies a substance whether it is in its liquid or. Does Solid Liquid Extraction Used Boiling Point.
From www.slideserve.com
PPT Chapter 13 continued… PowerPoint Presentation, free download ID Does Solid Liquid Extraction Used Boiling Point The biggest difference between these two processes is this: Solvent partitioning requires two solvents that are not miscible in each other. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). It purifies a substance whether it. Does Solid Liquid Extraction Used Boiling Point.
From www.researchgate.net
Schematic representation of different extraction methods solidliquid Does Solid Liquid Extraction Used Boiling Point Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). It purifies a substance whether it is in its liquid or solid phase. Solvent partitioning requires two solvents that are. Does Solid Liquid Extraction Used Boiling Point.
From www.youtube.com
BOILING POINT OF LIQUIDS YouTube Does Solid Liquid Extraction Used Boiling Point The biggest difference between these two processes is this: The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). It purifies a substance whether it is in its liquid or solid phase. Solvent partitioning requires two solvents. Does Solid Liquid Extraction Used Boiling Point.
From www.youtube.com
Binary Boiling Point Diagram of a LiquidLiquid Mixture YouTube Does Solid Liquid Extraction Used Boiling Point It purifies a substance whether it is in its liquid or solid phase. It purifies a substance only in its liquid phase. The biggest difference between these two processes is this: Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most. Does Solid Liquid Extraction Used Boiling Point.
From www.slideserve.com
PPT SolidLiquid Extraction Solid liquid extraction(leaching) means Does Solid Liquid Extraction Used Boiling Point It purifies a substance only in its liquid phase. Solvent partitioning requires two solvents that are not miscible in each other. Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply. Does Solid Liquid Extraction Used Boiling Point.
From thepiquelab.com
Tackling Heat Energy Questions Melting & Boiling Points! Primary Does Solid Liquid Extraction Used Boiling Point The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). Solvent partitioning requires two solvents that are not miscible in each other. Usually one of the solvents is water. The biggest difference between these two processes is. Does Solid Liquid Extraction Used Boiling Point.
From studylib.net
Boiling Point = Temperature at which a liquid turns into a gas Does Solid Liquid Extraction Used Boiling Point Usually one of the solvents is water. The biggest difference between these two processes is this: The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). It purifies a substance only in its liquid phase. It purifies. Does Solid Liquid Extraction Used Boiling Point.
From www.youtube.com
Liquid Liquid Extraction Process What is Solvent Extraction Process Does Solid Liquid Extraction Used Boiling Point Usually one of the solvents is water. The biggest difference between these two processes is this: It purifies a substance only in its liquid phase. It purifies a substance whether it is in its liquid or solid phase. Solvent partitioning requires two solvents that are not miscible in each other. The other solvent is a liquid that does not dissolve. Does Solid Liquid Extraction Used Boiling Point.
From owlcation.com
What Are the Freezing, Melting, and Boiling Points of Solids, Liquids Does Solid Liquid Extraction Used Boiling Point The biggest difference between these two processes is this: It purifies a substance whether it is in its liquid or solid phase. Solvent partitioning requires two solvents that are not miscible in each other. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether,. Does Solid Liquid Extraction Used Boiling Point.
From chem.libretexts.org
1A.3 Classifying Matter Chemistry LibreTexts Does Solid Liquid Extraction Used Boiling Point The biggest difference between these two processes is this: Usually one of the solvents is water. It purifies a substance whether it is in its liquid or solid phase. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called. Does Solid Liquid Extraction Used Boiling Point.
From www.gerhardt.de
Solidliquid extractions in fat analysis Does Solid Liquid Extraction Used Boiling Point Usually one of the solvents is water. It purifies a substance whether it is in its liquid or solid phase. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). It purifies a substance only in its. Does Solid Liquid Extraction Used Boiling Point.
From www.aurorabiomed.com
LiquidLiquid Extraction vs. SolidPhase Extraction Does Solid Liquid Extraction Used Boiling Point Solvent partitioning requires two solvents that are not miscible in each other. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). It purifies a substance only in its liquid phase. Usually one of the solvents is. Does Solid Liquid Extraction Used Boiling Point.
From www.researchgate.net
Schematic representation of the solidliquid extraction technique for Does Solid Liquid Extraction Used Boiling Point It purifies a substance only in its liquid phase. The biggest difference between these two processes is this: The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). Solvent partitioning requires two solvents that are not miscible. Does Solid Liquid Extraction Used Boiling Point.
From favpng.com
Boiling Point Melting Point Heat Temperature Chemistry, PNG Does Solid Liquid Extraction Used Boiling Point It purifies a substance whether it is in its liquid or solid phase. The biggest difference between these two processes is this: The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). Solvent partitioning requires two solvents. Does Solid Liquid Extraction Used Boiling Point.
From www.youtube.com
Separation of solidliquid mixtures YouTube Does Solid Liquid Extraction Used Boiling Point It purifies a substance only in its liquid phase. Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). The biggest difference between these two processes is this: Solvent partitioning. Does Solid Liquid Extraction Used Boiling Point.
From www.extractionforplants.com
7 Key Points Guide to SolidLiquid Extraction Process Does Solid Liquid Extraction Used Boiling Point The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). The biggest difference between these two processes is this: Solvent partitioning requires two solvents that are not miscible in each other. It purifies a substance only in. Does Solid Liquid Extraction Used Boiling Point.
From general.chemistrysteps.com
Entropy and State Change Chemistry Steps Does Solid Liquid Extraction Used Boiling Point It purifies a substance only in its liquid phase. Solvent partitioning requires two solvents that are not miscible in each other. The biggest difference between these two processes is this: The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often. Does Solid Liquid Extraction Used Boiling Point.
From www.youtube.com
Solid Liquid Extraction YouTube Does Solid Liquid Extraction Used Boiling Point Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). It purifies a substance whether it is in its liquid or solid phase. The biggest difference between these two processes. Does Solid Liquid Extraction Used Boiling Point.
From www.youtube.com
Method To Determine Boiling Point Of A Liquid Basic Principles and Does Solid Liquid Extraction Used Boiling Point It purifies a substance only in its liquid phase. Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). The biggest difference between these two processes is this: Solvent partitioning. Does Solid Liquid Extraction Used Boiling Point.
From chem.libretexts.org
10.4 Properties of Liquids Chemistry LibreTexts Does Solid Liquid Extraction Used Boiling Point Solvent partitioning requires two solvents that are not miscible in each other. It purifies a substance whether it is in its liquid or solid phase. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). It purifies. Does Solid Liquid Extraction Used Boiling Point.
From preparatorychemistry.com
pH and Equilibrium Does Solid Liquid Extraction Used Boiling Point The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). Solvent partitioning requires two solvents that are not miscible in each other. It purifies a substance whether it is in its liquid or solid phase. Usually one. Does Solid Liquid Extraction Used Boiling Point.
From www.britannica.com
distillation summary Britannica Does Solid Liquid Extraction Used Boiling Point The biggest difference between these two processes is this: It purifies a substance only in its liquid phase. Solvent partitioning requires two solvents that are not miscible in each other. It purifies a substance whether it is in its liquid or solid phase. The other solvent is a liquid that does not dissolve very well in water, such as diethyl. Does Solid Liquid Extraction Used Boiling Point.
From www.slideserve.com
PPT Extraction PowerPoint Presentation, free download ID9347367 Does Solid Liquid Extraction Used Boiling Point The biggest difference between these two processes is this: Usually one of the solvents is water. Solvent partitioning requires two solvents that are not miscible in each other. It purifies a substance only in its liquid phase. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common. Does Solid Liquid Extraction Used Boiling Point.
From mavink.com
Solid Liquid Extraction Does Solid Liquid Extraction Used Boiling Point It purifies a substance only in its liquid phase. It purifies a substance whether it is in its liquid or solid phase. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). Solvent partitioning requires two solvents. Does Solid Liquid Extraction Used Boiling Point.
From mavink.com
Solid Liquid Extraction Does Solid Liquid Extraction Used Boiling Point The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). It purifies a substance whether it is in its liquid or solid phase. The biggest difference between these two processes is this: Solvent partitioning requires two solvents. Does Solid Liquid Extraction Used Boiling Point.
From www.researchgate.net
Diagrammatic illustration of liquidliquid extraction (adapted from Does Solid Liquid Extraction Used Boiling Point The biggest difference between these two processes is this: It purifies a substance only in its liquid phase. Solvent partitioning requires two solvents that are not miscible in each other. Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common. Does Solid Liquid Extraction Used Boiling Point.
From www.researchgate.net
2 The schematic diagram of LiquidLiquid Extraction. The figure is Does Solid Liquid Extraction Used Boiling Point Usually one of the solvents is water. Solvent partitioning requires two solvents that are not miscible in each other. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). It purifies a substance only in its liquid. Does Solid Liquid Extraction Used Boiling Point.
From www.animalia-life.club
Industrial Liquid Liquid Extraction Does Solid Liquid Extraction Used Boiling Point The biggest difference between these two processes is this: Usually one of the solvents is water. Solvent partitioning requires two solvents that are not miscible in each other. It purifies a substance whether it is in its liquid or solid phase. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this. Does Solid Liquid Extraction Used Boiling Point.
From www.youtube.com
Boiling Points of Liquids, Chemistry Lecture Sabaq.pk YouTube Does Solid Liquid Extraction Used Boiling Point Solvent partitioning requires two solvents that are not miscible in each other. Usually one of the solvents is water. The biggest difference between these two processes is this: It purifies a substance only in its liquid phase. It purifies a substance whether it is in its liquid or solid phase. The other solvent is a liquid that does not dissolve. Does Solid Liquid Extraction Used Boiling Point.
From www.researchgate.net
Diagrammatic steps of solid phase extraction (adapted from Badawy et Does Solid Liquid Extraction Used Boiling Point The biggest difference between these two processes is this: The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). It purifies a substance only in its liquid phase. It purifies a substance whether it is in its. Does Solid Liquid Extraction Used Boiling Point.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting Does Solid Liquid Extraction Used Boiling Point The biggest difference between these two processes is this: The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). It purifies a substance only in its liquid phase. Usually one of the solvents is water. Solvent partitioning. Does Solid Liquid Extraction Used Boiling Point.
From www.youtube.com
Separating Liquids by Distillation YouTube Does Solid Liquid Extraction Used Boiling Point Usually one of the solvents is water. It purifies a substance whether it is in its liquid or solid phase. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). The biggest difference between these two processes. Does Solid Liquid Extraction Used Boiling Point.
From www.dreamstime.com
Fractional Distillation is a Process Used To Separate a Mixture of Two Does Solid Liquid Extraction Used Boiling Point The biggest difference between these two processes is this: It purifies a substance only in its liquid phase. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). Usually one of the solvents is water. It purifies. Does Solid Liquid Extraction Used Boiling Point.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Does Solid Liquid Extraction Used Boiling Point It purifies a substance whether it is in its liquid or solid phase. It purifies a substance only in its liquid phase. Solvent partitioning requires two solvents that are not miscible in each other. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether,. Does Solid Liquid Extraction Used Boiling Point.