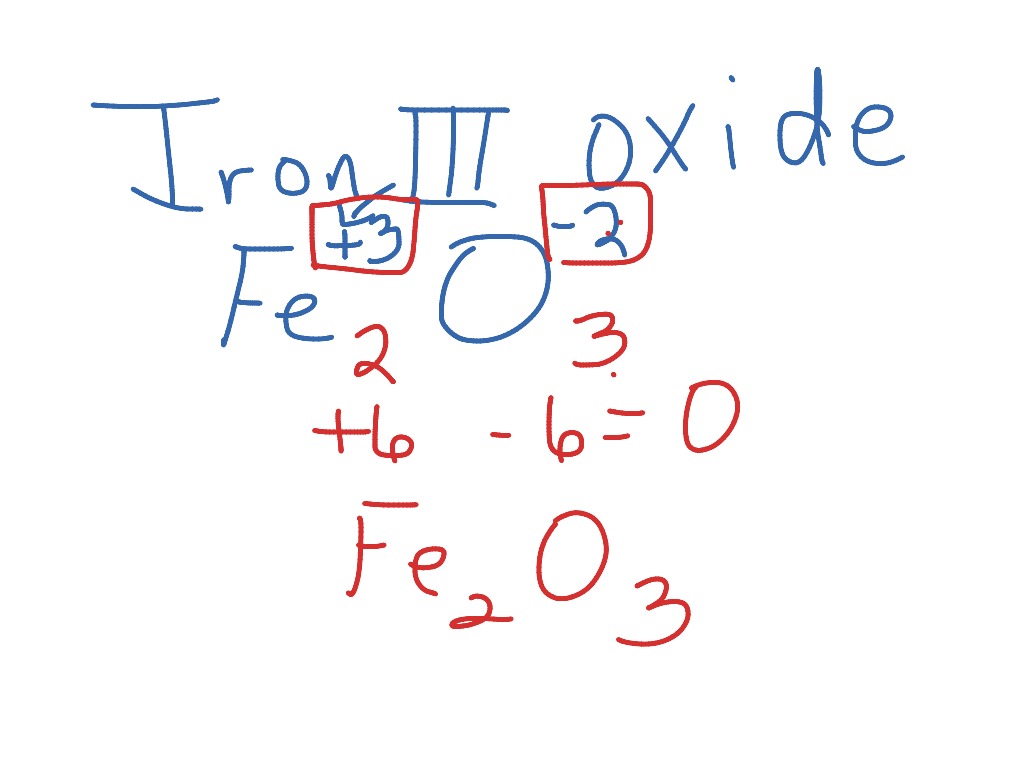Iron (Iii) Oxide Trihydrate Formula . The chemical equation for this reaction is: Iron (iii) oxide is a compound that appears in at least four different polymorphs: Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the following. Oxidation is the process in which an atom loses an electron. It is formed when iron undergoes oxidation. So fe +2 is iron (ii) and fe +3 is iron (iii). Iron oxide (ii,iii), magnetic nanoparticles solution. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}.
from www.showme.com
2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. The chemical equation for this reaction is: Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the following. So fe +2 is iron (ii) and fe +3 is iron (iii). Iron oxide (ii,iii), magnetic nanoparticles solution. Iron (iii) oxide is a compound that appears in at least four different polymorphs: Oxidation is the process in which an atom loses an electron. Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide.
Iron III oxide Science, Chemistry, Chemical Bonds ShowMe
Iron (Iii) Oxide Trihydrate Formula Iron oxide (ii,iii), magnetic nanoparticles solution. The chemical equation for this reaction is: Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. Iron (iii) oxide is a compound that appears in at least four different polymorphs: Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Iron oxide (ii,iii), magnetic nanoparticles solution. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Oxidation is the process in which an atom loses an electron. So fe +2 is iron (ii) and fe +3 is iron (iii). Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the following. It is formed when iron undergoes oxidation. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Iron (Iii) Oxide Trihydrate Formula 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Oxidation is the process in which an atom loses an electron. It is formed when iron undergoes oxidation. Iron (iii) oxide is. Iron (Iii) Oxide Trihydrate Formula.
From www.numerade.com
SOLVED write the formula for iron(III) fluoride trihydrate Iron (Iii) Oxide Trihydrate Formula The chemical equation for this reaction is: Oxidation is the process in which an atom loses an electron. Iron oxide (ii,iii), magnetic nanoparticles solution. It is formed when iron undergoes oxidation. So fe +2 is iron (ii) and fe +3 is iron (iii). Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Both methods result in the formation. Iron (Iii) Oxide Trihydrate Formula.
From stock.adobe.com
iron III Oxide Properties and Chemical Compound Structure Stock Vector Iron (Iii) Oxide Trihydrate Formula 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Iron oxide (ii,iii), magnetic nanoparticles solution. Iron (iii) oxide is a compound that appears in at least four different polymorphs: Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. The chemical equation for this reaction is: Oxidation is the process in which an atom. Iron (Iii) Oxide Trihydrate Formula.
From www.slideserve.com
PPT Naming Type (I) and (II) Compounds PowerPoint Presentation, free Iron (Iii) Oxide Trihydrate Formula It is formed when iron undergoes oxidation. The chemical equation for this reaction is: Iron oxide (ii,iii), magnetic nanoparticles solution. Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Iron (iii) oxide is a compound that appears in at least four different polymorphs: Both methods result in the formation of iron (iii) oxide, commonly used. Iron (Iii) Oxide Trihydrate Formula.
From www.showme.com
Iron III oxide Science, Chemistry, Chemical Bonds ShowMe Iron (Iii) Oxide Trihydrate Formula Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. The chemical equation for this reaction is: Iron oxide (ii,iii), magnetic nanoparticles solution. It is formed when iron undergoes oxidation. Some of the metals form very common ions which have latin names that are in common use, and you need to. Iron (Iii) Oxide Trihydrate Formula.
From www.shutterstock.com
Ironiii Oxide Ferric Oxide Compound 스톡 일러스트 1073727407 Iron (Iii) Oxide Trihydrate Formula Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Oxidation is the process in which an atom loses an electron. Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the following. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. So fe +2 is. Iron (Iii) Oxide Trihydrate Formula.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID2435173 Iron (Iii) Oxide Trihydrate Formula 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. It is formed when iron undergoes oxidation. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Iron oxide (ii,iii), magnetic nanoparticles solution. Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. Oxidation is the process in which an atom loses an electron. Iron. Iron (Iii) Oxide Trihydrate Formula.
From webgiasi.vn
How to Calculate the Molar Mass / Molecular Weight of Fe2O3 — Iron (III Iron (Iii) Oxide Trihydrate Formula Iron (iii) oxide is a compound that appears in at least four different polymorphs: Oxidation is the process in which an atom loses an electron. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. Some of the metals form very common ions which have latin names that are in common. Iron (Iii) Oxide Trihydrate Formula.
From www.toppr.com
Iron III Oxide Formula Definition, Concepts and Examples Iron (Iii) Oxide Trihydrate Formula So fe +2 is iron (ii) and fe +3 is iron (iii). Oxidation is the process in which an atom loses an electron. Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. It is formed when iron undergoes oxidation. The chemical equation for this reaction is: Some of the metals form very common ions which have latin names that are in. Iron (Iii) Oxide Trihydrate Formula.
From www.nanochemazone.com
Ammonium Iron(III) Oxalate Trihydrate Powder Low Price 1 Nanochemazone Iron (Iii) Oxide Trihydrate Formula The chemical equation for this reaction is: It is formed when iron undergoes oxidation. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. So fe +2 is iron (ii) and fe. Iron (Iii) Oxide Trihydrate Formula.
From www.pw.live
Iron III Oxide Formula, Structure, Properties, Uses Iron (Iii) Oxide Trihydrate Formula It is formed when iron undergoes oxidation. The chemical equation for this reaction is: Iron oxide (ii,iii), magnetic nanoparticles solution. Iron (iii) oxide is a compound that appears in at least four different polymorphs: So fe +2 is iron (ii) and fe +3 is iron (iii). Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Magnetic iron oxide. Iron (Iii) Oxide Trihydrate Formula.
From www.fishersci.es
Citrato de hierro(III) trihidrato, puro, Fisher Chemical Fisher Iron (Iii) Oxide Trihydrate Formula Iron oxide (ii,iii), magnetic nanoparticles solution. Oxidation is the process in which an atom loses an electron. It is formed when iron undergoes oxidation. The chemical equation for this reaction is: So fe +2 is iron (ii) and fe +3 is iron (iii). Some of the metals form very common ions which have latin names that are in common use,. Iron (Iii) Oxide Trihydrate Formula.
From www.biosynth.com
FA34409 13268423 Ammonium iron (III) oxalate trihydrate Iron (Iii) Oxide Trihydrate Formula Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the following. It is formed when iron undergoes oxidation. Iron (iii) oxide is. Iron (Iii) Oxide Trihydrate Formula.
From www.fishersci.ca
Iron(III) hydroxide, 99+, Thermo Scientific Chemicals Fisher Scientific Iron (Iii) Oxide Trihydrate Formula Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. So fe +2 is iron (ii) and fe +3 is iron (iii). It is formed when iron undergoes oxidation. The chemical equation for this reaction is: Some of the metals form very common ions which have latin names that are in common use, and you need. Iron (Iii) Oxide Trihydrate Formula.
From www.youtube.com
Formation of Rust Hydrated Iron (III) Oxide YouTube Iron (Iii) Oxide Trihydrate Formula Iron (iii) oxide is a compound that appears in at least four different polymorphs: So fe +2 is iron (ii) and fe +3 is iron (iii). Oxidation is the process in which an atom loses an electron. Iron oxide (ii,iii), magnetic nanoparticles solution. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments. Iron (Iii) Oxide Trihydrate Formula.
From www.youtube.com
How to write the formula for iron (III) oxide YouTube Iron (Iii) Oxide Trihydrate Formula Iron oxide (ii,iii), magnetic nanoparticles solution. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the following. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. So fe +2 is iron (ii) and fe +3. Iron (Iii) Oxide Trihydrate Formula.
From www.sigmaaldrich.com
Iron(III) oxide hydrated, catalyst grade, 3050 mesh SigmaAldrich Iron (Iii) Oxide Trihydrate Formula Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Iron oxide (ii,iii), magnetic nanoparticles solution. The chemical equation for this reaction is: 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. It is formed when iron undergoes oxidation. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Iron (iii). Iron (Iii) Oxide Trihydrate Formula.
From www.thoughtco.com
Easy Steps for Balancing Chemical Equations Iron (Iii) Oxide Trihydrate Formula Iron (iii) oxide is a compound that appears in at least four different polymorphs: Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. Iron oxide (ii,iii), magnetic nanoparticles solution. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. So fe +2 is iron (ii) and fe +3 is iron (iii). The chemical equation. Iron (Iii) Oxide Trihydrate Formula.
From www.youtube.com
What is the formula for iron III oxide? YouTube Iron (Iii) Oxide Trihydrate Formula Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the following. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. It is formed. Iron (Iii) Oxide Trihydrate Formula.
From www.numerade.com
SOLVED Calculate the mass of Iron(III) oxide (FezO;) that contains a Iron (Iii) Oxide Trihydrate Formula Iron oxide (ii,iii), magnetic nanoparticles solution. So fe +2 is iron (ii) and fe +3 is iron (iii). Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the following. Iron (iii) oxide has the chemical. Iron (Iii) Oxide Trihydrate Formula.
From slideplayer.com
Calculations Based on Chemical Equations ppt download Iron (Iii) Oxide Trihydrate Formula Iron (iii) oxide is a compound that appears in at least four different polymorphs: It is formed when iron undergoes oxidation. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Oxidation is the process in which an. Iron (Iii) Oxide Trihydrate Formula.
From www.numerade.com
SOLVED Iron reacts with oxygen to form iron(III) oxide according to Iron (Iii) Oxide Trihydrate Formula Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. So fe +2 is iron (ii) and fe +3 is iron (iii). Iron (iii) oxide is a compound that appears in at least four different polymorphs: Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in. Iron (Iii) Oxide Trihydrate Formula.
From inoxia.co.uk
Buy Iron (III) Oxide at Inoxia Ltd Iron (Iii) Oxide Trihydrate Formula The chemical equation for this reaction is: It is formed when iron undergoes oxidation. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Iron oxide (ii,iii), magnetic nanoparticles solution. Oxidation is the process in which an atom. Iron (Iii) Oxide Trihydrate Formula.
From www.showme.com
Write the formula for iron (III) oxide and iron (III) sulfate Iron (Iii) Oxide Trihydrate Formula Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the following. Oxidation is the process in which an atom loses an electron. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. The chemical equation for this reaction is:. Iron (Iii) Oxide Trihydrate Formula.
From www.sciencephoto.com
Iron(III) Oxide Stock Image C030/8153 Science Photo Library Iron (Iii) Oxide Trihydrate Formula Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. The chemical equation for this reaction is: Oxidation is the process in which an atom loses an electron. Iron (iii) oxide is a compound that appears in at least four different polymorphs: Some of the metals form very common. Iron (Iii) Oxide Trihydrate Formula.
From www.numerade.com
SOLVEDThe formation of rust [Iron (III) oxide] on the surface of iron Iron (Iii) Oxide Trihydrate Formula It is formed when iron undergoes oxidation. The chemical equation for this reaction is: So fe +2 is iron (ii) and fe +3 is iron (iii). Iron oxide (ii,iii), magnetic nanoparticles solution. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Some of the metals form very common ions which have latin names that are in common use, and you need to be. Iron (Iii) Oxide Trihydrate Formula.
From www.chegg.com
Solved 6. Iron reacts with oxygen gas to form iron (III) Iron (Iii) Oxide Trihydrate Formula It is formed when iron undergoes oxidation. So fe +2 is iron (ii) and fe +3 is iron (iii). Iron oxide (ii,iii), magnetic nanoparticles solution. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Iron (iii) oxide. Iron (Iii) Oxide Trihydrate Formula.
From www.sigmaaldrich.co.th
AMMONIUM IRON(III) OXALATE TRIHYDRATE, > Merck Life Sciences Thailand Iron (Iii) Oxide Trihydrate Formula 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. So fe +2 is iron (ii) and fe +3 is iron (iii). The chemical equation for this reaction is: Oxidation is the process in which an atom loses an electron. It is formed when iron undergoes oxidation. Iron oxide (ii,iii), magnetic nanoparticles solution.. Iron (Iii) Oxide Trihydrate Formula.
From www.slideserve.com
PPT 10 pt PowerPoint Presentation, free download ID5741064 Iron (Iii) Oxide Trihydrate Formula 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. So fe +2 is iron (ii) and fe +3 is iron (iii). Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. Oxidation is the process in which an atom loses an electron. Iron oxide (ii,iii), magnetic nanoparticles solution. It is formed when iron undergoes. Iron (Iii) Oxide Trihydrate Formula.
From testbook.com
Iron(III) Oxide Formula Know Its Preparation, Properties & Uses Iron (Iii) Oxide Trihydrate Formula Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Iron (iii) oxide is a compound that appears in at least four different polymorphs: Oxidation is the process in which an atom loses an electron. It is formed when iron undergoes oxidation. Iron (iii) oxide has the chemical formula. Iron (Iii) Oxide Trihydrate Formula.
From slideplayer.com
Ionic Compounds Naming ppt download Iron (Iii) Oxide Trihydrate Formula So fe +2 is iron (ii) and fe +3 is iron (iii). Iron oxide (ii,iii), magnetic nanoparticles solution. It is formed when iron undergoes oxidation. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. The chemical equation for this reaction is: Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron. Iron (Iii) Oxide Trihydrate Formula.
From www.shutterstock.com
Iron Iii Hydroxide Formula Handwritten Chemical Stock Illustration Iron (Iii) Oxide Trihydrate Formula 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. The chemical equation for this reaction is: Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. It is formed when iron undergoes oxidation. Magnetic iron oxide nanocrystals, magnetite, superparamagnetic iron oxide. So fe +2 is iron (ii) and fe +3 is iron (iii). Oxidation is the process in which an atom loses. Iron (Iii) Oxide Trihydrate Formula.
From slideplayer.com
Calculations Based on Chemical Equations ppt download Iron (Iii) Oxide Trihydrate Formula It is formed when iron undergoes oxidation. Iron oxide (ii,iii), magnetic nanoparticles solution. So fe +2 is iron (ii) and fe +3 is iron (iii). Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with. Iron (Iii) Oxide Trihydrate Formula.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas Bubbles Of Hydrogen Gas And Aqueous Iron (iii) Iron (Iii) Oxide Trihydrate Formula Iron oxide (ii,iii), magnetic nanoparticles solution. So fe +2 is iron (ii) and fe +3 is iron (iii). Iron (iii) oxide is a compound that appears in at least four different polymorphs: It is formed when iron undergoes oxidation. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Both methods result in the formation of iron (iii) oxide,. Iron (Iii) Oxide Trihydrate Formula.
From www.youtube.com
How to Write the Formula for Iron (III) Oxide YouTube Iron (Iii) Oxide Trihydrate Formula Oxidation is the process in which an atom loses an electron. It is formed when iron undergoes oxidation. Iron oxide (ii,iii), magnetic nanoparticles solution. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. So fe +2 is iron (ii) and fe +3 is iron (iii). Both methods result in the formation of iron (iii) oxide, commonly used in. Iron (Iii) Oxide Trihydrate Formula.