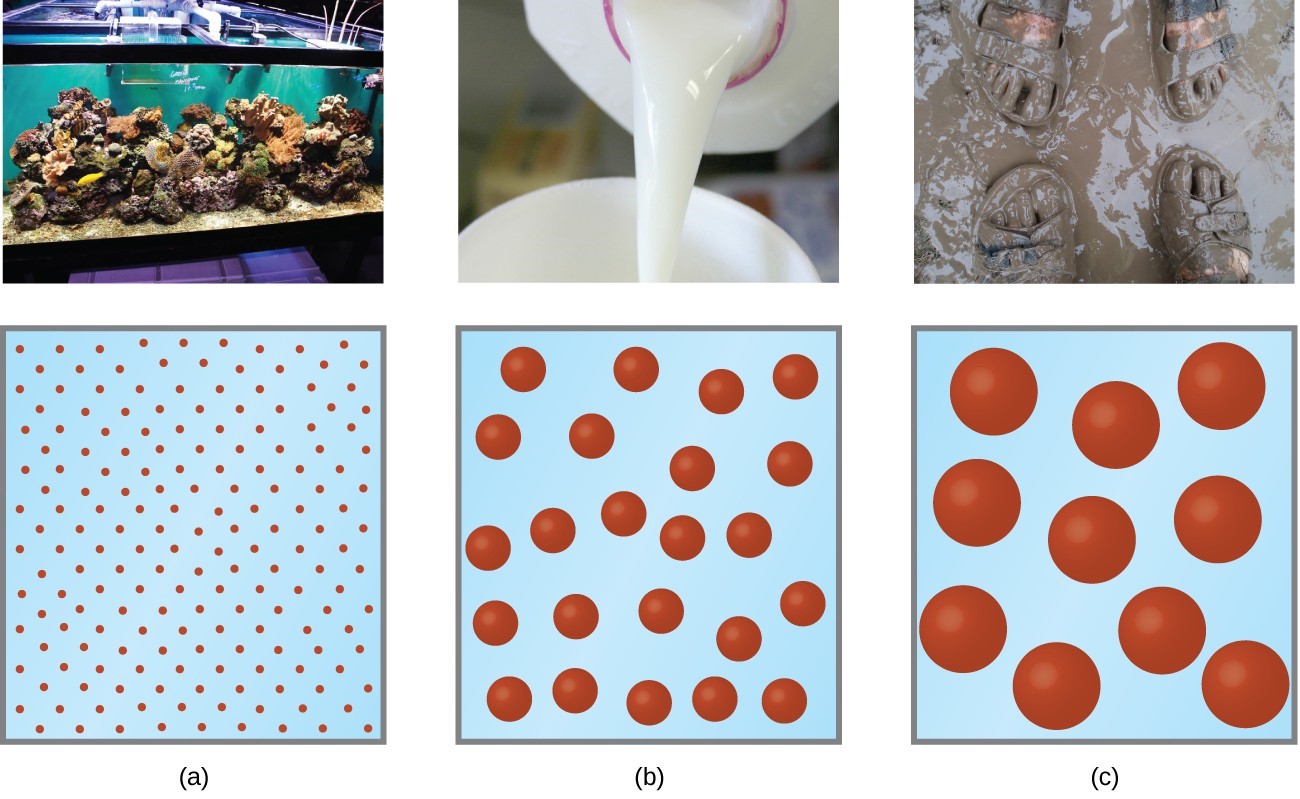
Colloid Solution N . Unlike true solutions where solute. These particles can be solid, liquid, or gas. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. For the various dispersion types (emulsion, gel, sol, foam, etc.),. Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium.
from uen.pressbooks.pub
In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Unlike true solutions where solute. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. For the various dispersion types (emulsion, gel, sol, foam, etc.),. These particles can be solid, liquid, or gas. Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium.
Colloids Introductory Chemistry
Colloid Solution N These particles can be solid, liquid, or gas. These particles can be solid, liquid, or gas. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. For the various dispersion types (emulsion, gel, sol, foam, etc.),. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Unlike true solutions where solute.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Colloid Solution N For the various dispersion types (emulsion, gel, sol, foam, etc.),. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Unlike true solutions where solute. A colloid is a heterogeneous mixture whose. Colloid Solution N.
From www.animalia-life.club
Colloid Milk Colloid Solution N Unlike true solutions where solute. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A colloid is. Colloid Solution N.
From nurseslabs.com
IV Fluids and Solutions Guide & Cheat Sheet (2023 Update) Nurseslabs Colloid Solution N These particles can be solid, liquid, or gas. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. For the various dispersion types (emulsion, gel, sol, foam, etc.),. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Unlike. Colloid Solution N.
From www.youtube.com
Science Quiz Solution, Suspension or Colloid ANY 10 YouTube Colloid Solution N A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Unlike true solutions where solute. A colloid is. Colloid Solution N.
From brainly.in
what is a colloidal solution? Brainly.in Colloid Solution N The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Colloids are prepared by producing particles. Colloid Solution N.
From gionidedc.blob.core.windows.net
Colloid Solution In at Martin Miller blog Colloid Solution N These particles can be solid, liquid, or gas. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A colloid is a heterogeneous mixture whose particle size is intermediate between those. Colloid Solution N.
From www.slideserve.com
PPT Colloids, Solutions, Suspensions PowerPoint Presentation, free Colloid Solution N These particles can be solid, liquid, or gas. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Unlike true solutions where solute. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. In chemistry, a colloid is a mixture of tiny particles that are. Colloid Solution N.
From sciencenotes.org
What Is a Colloid? Definition and Examples Colloid Solution N In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. These particles can be solid, liquid, or gas. Unlike true solutions where solute. A colloidal solution typically consists of particles. Colloid Solution N.
From www.animalia-life.club
Colloid Milk Colloid Solution N In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout. Colloid Solution N.
From www.youtube.com
Solution Suspension Colloid YouTube Colloid Solution N These particles can be solid, liquid, or gas. Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. In chemistry,. Colloid Solution N.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Colloid Solution N The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. Unlike true solutions where solute. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. For the various dispersion types (emulsion, gel, sol, foam, etc.),. Colloids are prepared by producing. Colloid Solution N.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Colloid Solution N The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. These particles can be solid, liquid, or gas. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium.. Colloid Solution N.
From uen.pressbooks.pub
Colloids Introductory Chemistry Colloid Solution N These particles can be solid, liquid, or gas. For the various dispersion types (emulsion, gel, sol, foam, etc.),. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Unlike true solutions where solute. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Colloids are prepared by producing. Colloid Solution N.
From brainly.in
How are colloids and suspensions different from solutions ? Brainly.in Colloid Solution N A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium. Unlike true solutions where solute. These particles can be solid, liquid, or gas. For the various dispersion types (emulsion, gel, sol, foam, etc.),. Summarize the principal distinguishing. Colloid Solution N.
From pediaa.com
Difference Between Colloid and Solution Definition, Properties Colloid Solution N Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium. Unlike true solutions where solute. These particles can be solid, liquid, or gas. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. In chemistry, a colloid is a mixture of tiny particles that. Colloid Solution N.
From brainly.ph
Directions Study the pictures of mixtures below and classify whether Colloid Solution N A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. These particles can be solid, liquid, or gas. Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout. Colloid Solution N.
From suspensioncolloid-00.blogspot.com
91 SOLUTION COLLOID SUSPENSION YOUTUBE SuspensionColloid Colloid Solution N Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Unlike true solutions where solute. For the. Colloid Solution N.
From byjus.com
Classification of Colloids Dispersed Phase & Dispersion Medium Colloid Solution N In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Summarize the principal distinguishing properties of solutions, colloidal dispersions,. Colloid Solution N.
From alanabbaguilar.blogspot.com
Crystalloid and Colloid Fluids AlanabbAguilar Colloid Solution N A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Unlike true solutions where solute. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. In this chapter, we will consider the nature of solutions,. Colloid Solution N.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Colloid Solution N A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. For the various dispersion types (emulsion, gel, sol, foam, etc.),. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Colloids are prepared by producing particles of colloidal dimensions and distributing. Colloid Solution N.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Colloid Solution N Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. Unlike true solutions where solute. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium. A solution may be colored,. Colloid Solution N.
From www.nagwa.com
Vidéo de la leçon Colloïdes et les suspensions Nagwa Colloid Solution N The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Unlike true solutions where solute. In this chapter, we will consider the nature of solutions, and examine factors that determine. Colloid Solution N.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Colloid Solution N For the various dispersion types (emulsion, gel, sol, foam, etc.),. Unlike true solutions where solute. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. In this chapter, we will consider. Colloid Solution N.
From www.youtube.com
Solution colloids n suspension class 9 by neelam dwivedi YouTube Colloid Solution N In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. These particles can be solid, liquid, or gas. For the various dispersion types (emulsion, gel, sol, foam, etc.),. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. A colloid is a. Colloid Solution N.
From www.slideserve.com
PPT Colloids PowerPoint Presentation ID7036943 Colloid Solution N Unlike true solutions where solute. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. The particles are. Colloid Solution N.
From www.vecteezy.com
peptisation, conversion of freshly prepared precipitate into colloid by Colloid Solution N In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1. Colloid Solution N.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Colloid Solution N Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. These particles can be solid, liquid, or gas. Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium. A colloidal solution typically consists of particles ranging in size from. Colloid Solution N.
From www.theplasticsfella.com
Fluid Resuscitation in Burns · Formulas, Indications & Fluids Colloid Solution N In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. These particles can be solid, liquid, or gas. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Colloids are prepared by producing particles of colloidal dimensions and distributing. Colloid Solution N.
From fyosyximi.blob.core.windows.net
Colloid Solution And Suspension at Kristine Whitaker blog Colloid Solution N These particles can be solid, liquid, or gas. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Unlike true solutions where solute. Colloids are prepared by producing particles of colloidal dimensions. Colloid Solution N.
From www.slideserve.com
PPT Solutions and Colloidal Dispersions PowerPoint Presentation, free Colloid Solution N Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. These particles can be solid, liquid, or gas.. Colloid Solution N.
From suspensioncolloid-00.blogspot.com
89 SOLUTION SUSPENSION COLLOID PPT SuspensionColloid Colloid Solution N Unlike true solutions where solute. Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A colloid is a heterogeneous mixture whose particle size. Colloid Solution N.
From www.slideshare.net
Colloids Colloid Solution N The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a. Colloid Solution N.
From www.vrogue.co
What Is A Colloid Definition And Examples vrogue.co Colloid Solution N These particles can be solid, liquid, or gas. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium. For the various dispersion types (emulsion, gel, sol, foam, etc.),. A solution may. Colloid Solution N.
From pt.slideshare.net
Solutions, suspensions, and colloids Colloid Solution N For the various dispersion types (emulsion, gel, sol, foam, etc.),. These particles can be solid, liquid, or gas. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. A. Colloid Solution N.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Colloid Solution N These particles can be solid, liquid, or gas. Unlike true solutions where solute. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. For the various dispersion types (emulsion, gel, sol, foam, etc.),. Colloids are prepared by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium. A. Colloid Solution N.