Copper Electron Affinity . Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. Most copper ore is mined or extracted as copper sulfides. The electron affinity of copper is 118.4 kj mol ‑1. An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. Finally, the resulting crude copper is purified by electrolysis involving. Copper is then obtained by smelting and leaching. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.
from elchoroukhost.net
Copper is then obtained by smelting and leaching. Finally, the resulting crude copper is purified by electrolysis involving. An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. Most copper ore is mined or extracted as copper sulfides. The electron affinity of copper is 118.4 kj mol ‑1. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.
Copper Periodic Table Protons Neutrons And Electrons Elcho Table
Copper Electron Affinity An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. The electron affinity of copper is 118.4 kj mol ‑1. Finally, the resulting crude copper is purified by electrolysis involving. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. Copper is then obtained by smelting and leaching. Most copper ore is mined or extracted as copper sulfides. An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.
From www.alamy.com
3d render of atom structure of copper isolated over white background Copper Electron Affinity The electron affinity of copper is 118.4 kj mol ‑1. Most copper ore is mined or extracted as copper sulfides. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Copper is then obtained by smelting and leaching. An atom of copper in. Copper Electron Affinity.
From www.alamy.com
Symbol and electron diagram for Copper illustration Stock Vector Image Copper Electron Affinity Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. Finally, the resulting crude copper is purified by electrolysis involving. Most copper ore is mined or extracted as copper sulfides. Copper is then obtained by smelting and leaching. 119 rows electron affinity is. Copper Electron Affinity.
From awesomehome.co
Copper Periodic Table Protons Neutrons And Electrons Awesome Home Copper Electron Affinity An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. Copper is then obtained by smelting and leaching. Finally, the resulting crude copper is purified by electrolysis involving. The electron affinity of copper is 118.4 kj mol ‑1. Most copper ore is mined or extracted as. Copper Electron Affinity.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Copper Electron Affinity 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. Most copper ore is mined or extracted as copper. Copper Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Copper Electron Affinity The electron affinity of copper is 118.4 kj mol ‑1. Finally, the resulting crude copper is purified by electrolysis involving. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Most copper ore is mined or extracted as copper sulfides. Electron affinity is. Copper Electron Affinity.
From ppt-online.org
The Periodic Table презентация онлайн Copper Electron Affinity 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Copper is then obtained by smelting and leaching. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to. Copper Electron Affinity.
From www.nuclear-power.com
Phosphorus Electron Affinity Electronegativity Ionization Energy Copper Electron Affinity Copper is then obtained by smelting and leaching. Most copper ore is mined or extracted as copper sulfides. The electron affinity of copper is 118.4 kj mol ‑1. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. An atom of copper in. Copper Electron Affinity.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Copper Electron Affinity Copper is then obtained by smelting and leaching. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated. Copper Electron Affinity.
From animalia-life.club
Radon Lewis Dot Structure Copper Electron Affinity Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. Most copper ore is mined or extracted as copper sulfides. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. Copper Electron Affinity.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electron Affinity Copper is then obtained by smelting and leaching. Most copper ore is mined or extracted as copper sulfides. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. An atom of copper in the gas phase, for example, gives off energy when it. Copper Electron Affinity.
From elchoroukhost.net
Copper Periodic Table Protons Neutrons And Electrons Elcho Table Copper Electron Affinity Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. An atom of copper in the. Copper Electron Affinity.
From sites.google.com
Electron Affinity Periodic Trends Copper Electron Affinity 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. Finally, the resulting crude copper is purified by electrolysis. Copper Electron Affinity.
From www.nuclear-power.com
Copper Electron Affinity Electronegativity Ionization Energy of Copper Electron Affinity The electron affinity of copper is 118.4 kj mol ‑1. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Copper is then obtained by smelting and leaching. An atom of copper in the gas phase, for example, gives off energy when it. Copper Electron Affinity.
From www.pearson.com
Periodic Trend Electron Affinity Practice Problems Channels for Pearson+ Copper Electron Affinity Copper is then obtained by smelting and leaching. The electron affinity of copper is 118.4 kj mol ‑1. Finally, the resulting crude copper is purified by electrolysis involving. Most copper ore is mined or extracted as copper sulfides. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell. Copper Electron Affinity.
From mydigitalkemistry.com
Electron Affinity Trends Chemistry Digital Kemistry Best Online Copper Electron Affinity Copper is then obtained by smelting and leaching. Most copper ore is mined or extracted as copper sulfides. An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. Finally, the resulting crude copper is purified by electrolysis involving. The electron affinity of copper is 118.4 kj. Copper Electron Affinity.
From socratic.org
Which following pairs of atoms, have a lower electron affinity? a) Ca,K Copper Electron Affinity Finally, the resulting crude copper is purified by electrolysis involving. Copper is then obtained by smelting and leaching. The electron affinity of copper is 118.4 kj mol ‑1. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. An atom of copper in. Copper Electron Affinity.
From sciencenotes.org
Copper Facts Cu or Atomic Number 29 Copper Electron Affinity Copper is then obtained by smelting and leaching. Finally, the resulting crude copper is purified by electrolysis involving. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. Most copper ore is mined or extracted as copper sulfides. The electron affinity of copper. Copper Electron Affinity.
From www.pinterest.co.kr
Electron Affinity Trend and Definition Electron affinity, Ionization Copper Electron Affinity Finally, the resulting crude copper is purified by electrolysis involving. Copper is then obtained by smelting and leaching. The electron affinity of copper is 118.4 kj mol ‑1. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. 119 rows electron affinity is. Copper Electron Affinity.
From www.mooramo.com
Ions of Transition Elements Mooramo Copper Electron Affinity Copper is then obtained by smelting and leaching. An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. Finally,. Copper Electron Affinity.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Electron Affinity An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. Copper is then obtained by smelting and leaching. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. The. Copper Electron Affinity.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper Electron Affinity Copper is then obtained by smelting and leaching. Finally, the resulting crude copper is purified by electrolysis involving. Most copper ore is mined or extracted as copper sulfides. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. An atom of copper in. Copper Electron Affinity.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Copper Electron Affinity Finally, the resulting crude copper is purified by electrolysis involving. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. The electron affinity of copper is 118.4 kj mol ‑1. Copper is then obtained by smelting and leaching. 119 rows electron affinity is. Copper Electron Affinity.
From mungfali.com
Electronegativity And Electron Affinity Copper Electron Affinity An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. The electron affinity of copper is 118.4 kj mol ‑1. Finally, the resulting crude copper is purified by electrolysis involving. Most copper ore is mined or extracted as copper sulfides. Electron affinity is defined as the. Copper Electron Affinity.
From docslib.org
(Zns) ELECTRON AFFINITY in COPPER INDIUM SULFIDE (CIS) BASED Copper Electron Affinity Copper is then obtained by smelting and leaching. Most copper ore is mined or extracted as copper sulfides. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Finally, the resulting crude copper is purified by electrolysis involving. An atom of copper in. Copper Electron Affinity.
From www.mooramo.com
Gaining and Losing Electrons Mooramo Copper Electron Affinity The electron affinity of copper is 118.4 kj mol ‑1. An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. Finally, the resulting crude copper is purified by electrolysis involving. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron. Copper Electron Affinity.
From slideplayer.com
Atomic Theory. ppt download Copper Electron Affinity 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. Finally, the resulting crude copper is purified by electrolysis. Copper Electron Affinity.
From material-properties.org
Copper Periodic Table and Atomic Properties Copper Electron Affinity Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. The electron affinity of copper is 118.4 kj mol ‑1. Most copper ore is mined or extracted as copper sulfides. 119 rows electron affinity is the amount of energy change (δe) that occurs. Copper Electron Affinity.
From www.webelements.com
Elements Periodic Table » Copper » properties of free atoms Copper Electron Affinity The electron affinity of copper is 118.4 kj mol ‑1. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. Finally, the resulting crude copper is purified by electrolysis involving. An atom of copper in the gas phase, for example, gives off energy. Copper Electron Affinity.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Periodic Table Element Comparison Compare Copper vs Aluminium Copper Electron Affinity Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. The electron affinity of copper is 118.4 kj mol ‑1. Copper is then obtained by smelting and leaching. Finally, the resulting crude copper is purified by electrolysis involving. 119 rows electron affinity is. Copper Electron Affinity.
From www.earthdate.org
Copper’s Superpower EarthDate Copper Electron Affinity Most copper ore is mined or extracted as copper sulfides. Copper is then obtained by smelting and leaching. Finally, the resulting crude copper is purified by electrolysis involving. The electron affinity of copper is 118.4 kj mol ‑1. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron. Copper Electron Affinity.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper Electron Affinity Finally, the resulting crude copper is purified by electrolysis involving. Most copper ore is mined or extracted as copper sulfides. An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in. Copper Electron Affinity.
From www.dreamstime.com
Electron of the Element Copper Stock Vector Illustration of Copper Electron Affinity The electron affinity of copper is 118.4 kj mol ‑1. An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. Most copper ore is mined or extracted as copper sulfides. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in. Copper Electron Affinity.
From gbu-taganskij.ru
Electron Affinity Definition, Chart Trend In Periodic, 47 OFF Copper Electron Affinity The electron affinity of copper is 118.4 kj mol ‑1. Finally, the resulting crude copper is purified by electrolysis involving. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Electron affinity is defined as the change in energy (in kj/mole) of a. Copper Electron Affinity.
From www.nuclear-power.com
Hydrogen Electron Affinity Electronegativity Ionization Energy of Copper Electron Affinity An atom of copper in the gas phase, for example, gives off energy when it gains an electron to form an ion of copper. Copper is then obtained by smelting and leaching. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. Most. Copper Electron Affinity.
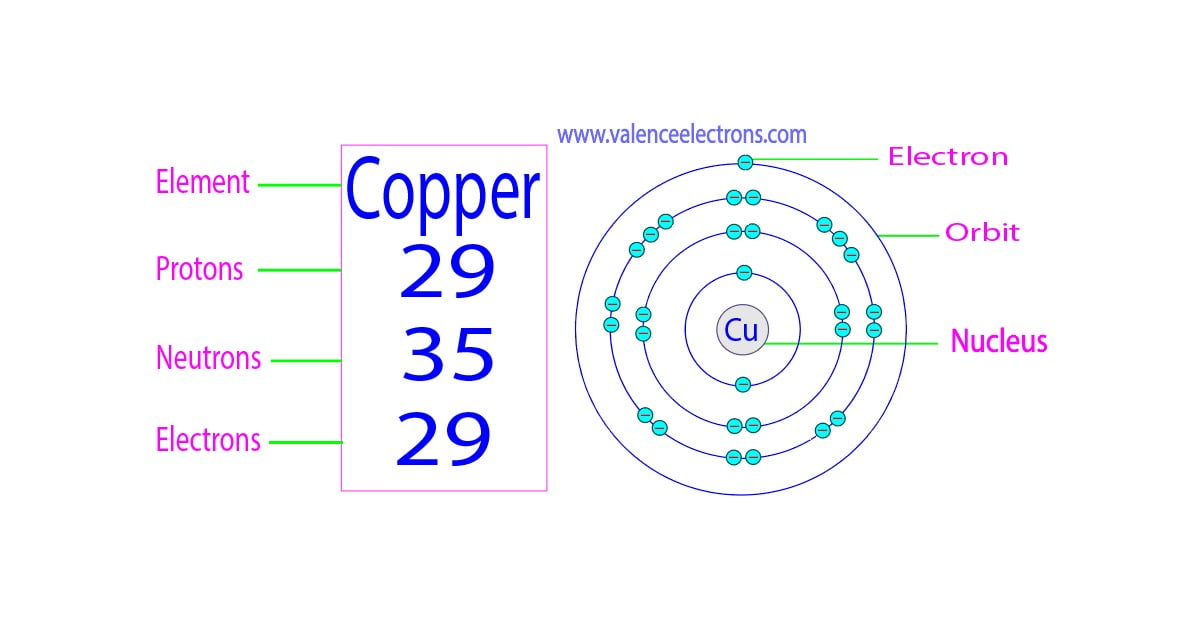
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Electron Affinity 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Most copper ore is mined or extracted as copper sulfides. Finally, the resulting crude copper is purified by electrolysis involving. The electron affinity of copper is 118.4 kj mol ‑1. Electron affinity is. Copper Electron Affinity.