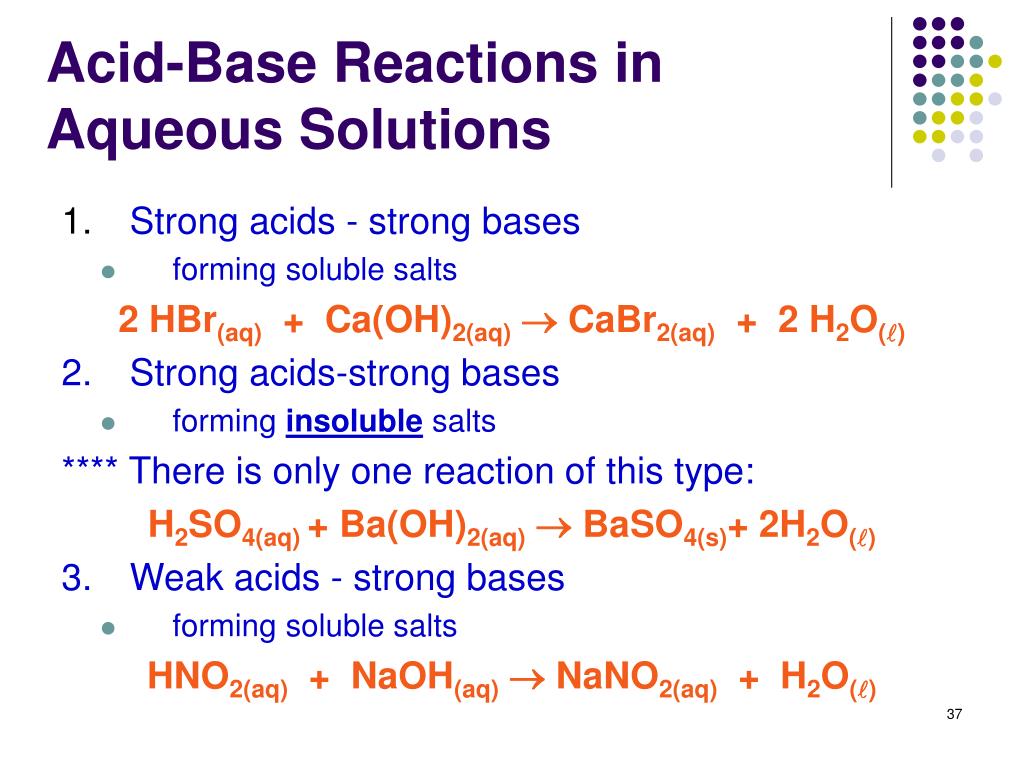
Weak Acid Strong Base Reaction Example . the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. mathematically prove that the ph at the halfway point of a titration of a weak acid with a strong base (where the volume of. Hc 2 h 3 o 2 is an. You correctly describe the strategy. any acid that dissociates 100% into ions is called a strong acid. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. The titration curve shown in figure 1 is for the titration of 25.00 ml of. If it does not dissociate 100%, it is a weak acid. The strategy of major species. Titration of a weak acid with a strong base. strong acids and bases are 100% ionized in aqueous solution. Weak acids and bases are less than 100%. one way of classifying acids and bases is as strong or weak:
from www.slideserve.com
These dissociate completely in water. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. any acid that dissociates 100% into ions is called a strong acid. strong acids and bases are 100% ionized in aqueous solution. You correctly describe the strategy. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. mathematically prove that the ph at the halfway point of a titration of a weak acid with a strong base (where the volume of. The strategy of major species. conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. one way of classifying acids and bases is as strong or weak:
PPT CHAPTER 10 Reactions in Aqueous Solutions I Acids, Bases & Salts
Weak Acid Strong Base Reaction Example one way of classifying acids and bases is as strong or weak: If it does not dissociate 100%, it is a weak acid. one way of classifying acids and bases is as strong or weak: Weak acids and bases are less than 100%. Titration of a weak acid with a strong base. strong acids and bases are 100% ionized in aqueous solution. The titration curve shown in figure 1 is for the titration of 25.00 ml of. mathematically prove that the ph at the halfway point of a titration of a weak acid with a strong base (where the volume of. The strategy of major species. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. any acid that dissociates 100% into ions is called a strong acid. conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. You correctly describe the strategy. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. These dissociate completely in water.
From www.khanacademy.org
Acidbase reactions (practice) Khan Academy Weak Acid Strong Base Reaction Example The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. The titration curve shown in figure 1 is for the titration of 25.00 ml of. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion.. Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT Chemical Reactions in Aqueous Solutions PowerPoint Presentation Weak Acid Strong Base Reaction Example You correctly describe the strategy. Titration of a weak acid with a strong base. conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. Hc 2 h 3 o 2 is an. Weak acids and bases are less than 100%. These dissociate completely in water.. Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT Weak Acids Weak Bases PowerPoint Presentation, free download ID Weak Acid Strong Base Reaction Example one way of classifying acids and bases is as strong or weak: conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Titration of. Weak Acid Strong Base Reaction Example.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases Weak Acid Strong Base Reaction Example The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. If it does not dissociate 100%, it is a weak acid. strong acids and. Weak Acid Strong Base Reaction Example.
From mavink.com
Acid Base Chemical Reaction Examples Weak Acid Strong Base Reaction Example If it does not dissociate 100%, it is a weak acid. These dissociate completely in water. You correctly describe the strategy. Weak acids and bases are less than 100%. conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. Hc 2 h 3 o 2. Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID Weak Acid Strong Base Reaction Example any acid that dissociates 100% into ions is called a strong acid. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. one way of classifying acids and bases is as strong or weak: Hc 2 h 3 o 2 is an. Weak acids and bases are less than 100%. The. Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT CHAPTER 10 Reactions in Aqueous Solutions I Acids, Bases & Salts Weak Acid Strong Base Reaction Example Hc 2 h 3 o 2 is an. If it does not dissociate 100%, it is a weak acid. The strategy of major species. Weak acids and bases are less than 100%. one way of classifying acids and bases is as strong or weak: These dissociate completely in water. the titration of a weak acid with a strong. Weak Acid Strong Base Reaction Example.
From www.teachoo.com
Examples of Weak Acids 5+ Examples Teachoo Teachoo Questions Weak Acid Strong Base Reaction Example The titration curve shown in figure 1 is for the titration of 25.00 ml of. mathematically prove that the ph at the halfway point of a titration of a weak acid with a strong base (where the volume of. Weak acids and bases are less than 100%. If it does not dissociate 100%, it is a weak acid. . Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2089759 Weak Acid Strong Base Reaction Example The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. strong acids and bases are 100% ionized in aqueous solution. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. These dissociate completely in water. Titration of a weak acid with a. Weak Acid Strong Base Reaction Example.
From www.teachoo.com
Salts and it's Properties (with Examples) Acids, Bases and Salt Weak Acid Strong Base Reaction Example The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. strong acids and bases are 100% ionized in aqueous solution. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. The strategy of major species. Hc 2 h 3 o 2 is. Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID Weak Acid Strong Base Reaction Example the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. The titration curve shown in figure 1 is for the titration of 25.00 ml of. You correctly describe. Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT Ch.9 J.C. Rowe PowerPoint Presentation, free download ID3493976 Weak Acid Strong Base Reaction Example The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. The strategy of major species. Weak acids and bases are less than 100%. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. You correctly describe the strategy. strong acids and bases. Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID Weak Acid Strong Base Reaction Example The strategy of major species. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The titration curve shown in figure 1 is for the titration of 25.00 ml of. The reaction of a strong acid with a strong base is a neutralization reaction, which. Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT AcidBase Reactions TITRATION PowerPoint Presentation, free Weak Acid Strong Base Reaction Example The titration curve shown in figure 1 is for the titration of 25.00 ml of. strong acids and bases are 100% ionized in aqueous solution. If it does not dissociate 100%, it is a weak acid. Hc 2 h 3 o 2 is an. conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak. Weak Acid Strong Base Reaction Example.
From dxolqhrho.blob.core.windows.net
What Happens When A Base Reacts With A Base at Larry Childers blog Weak Acid Strong Base Reaction Example strong acids and bases are 100% ionized in aqueous solution. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. The strategy of major species. Titration of a weak acid with a strong base. If it does not dissociate 100%, it is a weak acid. The reaction of a strong acid with. Weak Acid Strong Base Reaction Example.
From www.youtube.com
Weak acidweak base reactions Acids and bases AP Chemistry Khan Weak Acid Strong Base Reaction Example The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. The strategy of major species. mathematically prove that the ph at the halfway point of a titration of a weak acid with a strong base (where the volume of. any acid that dissociates 100% into ions is called a strong acid.. Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation ID6155898 Weak Acid Strong Base Reaction Example mathematically prove that the ph at the halfway point of a titration of a weak acid with a strong base (where the volume of. any acid that dissociates 100% into ions is called a strong acid. Titration of a weak acid with a strong base. one way of classifying acids and bases is as strong or weak:. Weak Acid Strong Base Reaction Example.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Weak Acid Strong Base Reaction Example The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. If it does not dissociate 100%, it is a weak acid. These dissociate completely in water. You correctly. Weak Acid Strong Base Reaction Example.
From www.teachoo.com
List of Strong Acids (More than 8 examples, with images) Teachoo Weak Acid Strong Base Reaction Example The strategy of major species. any acid that dissociates 100% into ions is called a strong acid. The titration curve shown in figure 1 is for the titration of 25.00 ml of. These dissociate completely in water. If it does not dissociate 100%, it is a weak acid. mathematically prove that the ph at the halfway point of. Weak Acid Strong Base Reaction Example.
From exoleqdka.blob.core.windows.net
Weak Acid Examples Ph at Melissa Morris blog Weak Acid Strong Base Reaction Example Weak acids and bases are less than 100%. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Titration of a weak acid with a strong base. Hc 2 h 3 o 2 is an. These dissociate completely in water. You correctly describe the strategy. one way of classifying. Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID Weak Acid Strong Base Reaction Example strong acids and bases are 100% ionized in aqueous solution. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Hc 2 h 3 o 2 is an. mathematically prove that the ph at the halfway point of a titration of a weak acid with a strong base. Weak Acid Strong Base Reaction Example.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Weak Acid Strong Base Reaction Example If it does not dissociate 100%, it is a weak acid. any acid that dissociates 100% into ions is called a strong acid. strong acids and bases are 100% ionized in aqueous solution. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. one way of classifying acids and bases. Weak Acid Strong Base Reaction Example.
From layton-blogcantrell.blogspot.com
Weak Base Examples Weak Acid Strong Base Reaction Example conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. any acid that dissociates 100% into ions is called a strong acid. Weak acids and bases are less than 100%. You correctly describe the strategy. The strategy of major species. the titration of. Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT Water The Universal Solvent PowerPoint Presentation, free Weak Acid Strong Base Reaction Example You correctly describe the strategy. mathematically prove that the ph at the halfway point of a titration of a weak acid with a strong base (where the volume of. Weak acids and bases are less than 100%. Hc 2 h 3 o 2 is an. one way of classifying acids and bases is as strong or weak: . Weak Acid Strong Base Reaction Example.
From www.teachoo.com
Examples of Weak Base (5 Examples with Images) Teachoo Chemistry Weak Acid Strong Base Reaction Example strong acids and bases are 100% ionized in aqueous solution. You correctly describe the strategy. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. mathematically prove that the ph at the halfway point of a titration of a weak acid with a strong base (where the volume. Weak Acid Strong Base Reaction Example.
From www.khanacademy.org
Acidbase reactions (practice) Khan Academy Weak Acid Strong Base Reaction Example Hc 2 h 3 o 2 is an. If it does not dissociate 100%, it is a weak acid. mathematically prove that the ph at the halfway point of a titration of a weak acid with a strong base (where the volume of. conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases. Weak Acid Strong Base Reaction Example.
From studytomikamariepc.z14.web.core.windows.net
How To Identify Acids And Bases In A Equation Weak Acid Strong Base Reaction Example strong acids and bases are 100% ionized in aqueous solution. Weak acids and bases are less than 100%. You correctly describe the strategy. conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. If it does not dissociate 100%, it is a weak acid.. Weak Acid Strong Base Reaction Example.
From www.youtube.com
Reaction weak acid Strong Base YouTube Weak Acid Strong Base Reaction Example The strategy of major species. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The titration curve shown in figure 1 is for the titration of 25.00 ml of. one way of classifying acids and bases is as strong or weak: Titration of. Weak Acid Strong Base Reaction Example.
From www.youtube.com
Weak acidstrong base reactions Acids and bases AP Chemistry Khan Weak Acid Strong Base Reaction Example You correctly describe the strategy. strong acids and bases are 100% ionized in aqueous solution. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a. Weak Acid Strong Base Reaction Example.
From www.youtube.com
net ionic equations strong bases and weak acids YouTube Weak Acid Strong Base Reaction Example strong acids and bases are 100% ionized in aqueous solution. Titration of a weak acid with a strong base. The strategy of major species. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. These dissociate completely in water. If it does not dissociate. Weak Acid Strong Base Reaction Example.
From ar.inspiredpencil.com
A Weak Base Strong Acid Weak Base With A Combines To Form Weak Acid Strong Base Reaction Example strong acids and bases are 100% ionized in aqueous solution. Titration of a weak acid with a strong base. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The titration curve shown in figure 1 is for the titration of 25.00 ml of.. Weak Acid Strong Base Reaction Example.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Weak Acid Strong Base Reaction Example If it does not dissociate 100%, it is a weak acid. You correctly describe the strategy. Weak acids and bases are less than 100%. The titration curve shown in figure 1 is for the titration of 25.00 ml of. The strategy of major species. strong acids and bases are 100% ionized in aqueous solution. one way of classifying. Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT Acid Base Reactions and Titration Curves PowerPoint Presentation Weak Acid Strong Base Reaction Example These dissociate completely in water. Weak acids and bases are less than 100%. conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. You correctly describe the strategy. one way of classifying acids and bases is as strong or weak: Hc 2 h 3. Weak Acid Strong Base Reaction Example.
From ar.inspiredpencil.com
A Weak Base Strong Acid Weak Base With A Combines To Form Weak Acid Strong Base Reaction Example Hc 2 h 3 o 2 is an. Titration of a weak acid with a strong base. The strategy of major species. conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. If it does not dissociate 100%, it is a weak acid. You correctly. Weak Acid Strong Base Reaction Example.
From www.slideserve.com
PPT Acids and bases, salts and solutions PowerPoint Presentation Weak Acid Strong Base Reaction Example You correctly describe the strategy. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. mathematically prove that the ph at the halfway point of a titration of a weak acid with a strong base (where the volume of. These dissociate completely in water. strong acids and bases are 100% ionized. Weak Acid Strong Base Reaction Example.