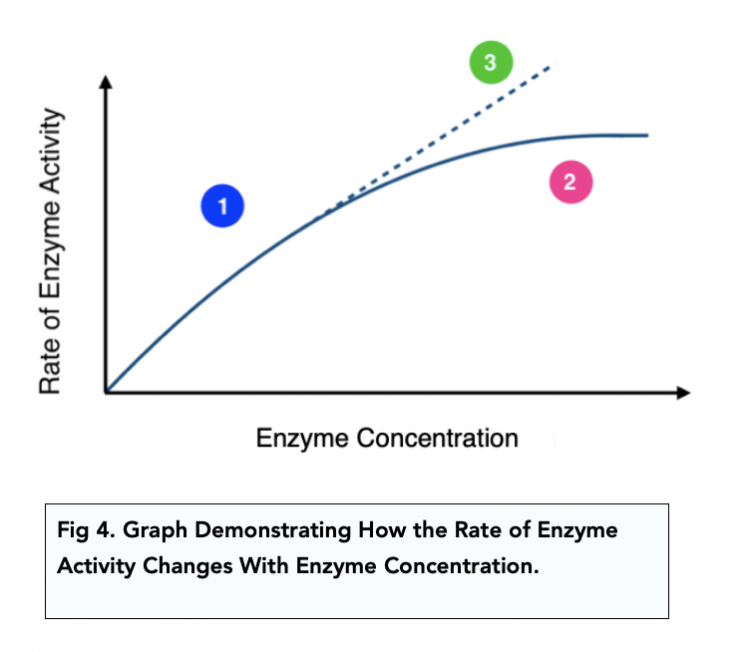
Enzymes Increase Keq . Biomolecules and the principles of enzyme kinetics and bioenergetics. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. In this case, the reaction favors the. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the reactants is large. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. In short, enzymes do not change the equilibrium state of a biochemical reaction. The second quarter deals mainly with intermediary metabolism. Δg 0 and k eq remain the same. Enzymes do not affect the thermodynamics of reactions. These products then become involved in some other biological pathway that initiates certain functions of the human body. This is known as the catalytic. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid.
from studymind.co.uk
For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. This is known as the catalytic. Biomolecules and the principles of enzyme kinetics and bioenergetics. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. These products then become involved in some other biological pathway that initiates certain functions of the human body. Enzymes do not affect the thermodynamics of reactions. In short, enzymes do not change the equilibrium state of a biochemical reaction.
Enzymes Rates of Reaction (Alevel Biology) Study Mind
Enzymes Increase Keq In short, enzymes do not change the equilibrium state of a biochemical reaction. This is known as the catalytic. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. In this case, the reaction favors the. Δg 0 and k eq remain the same. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. These products then become involved in some other biological pathway that initiates certain functions of the human body. The second quarter deals mainly with intermediary metabolism. Enzymes do not affect the thermodynamics of reactions. In short, enzymes do not change the equilibrium state of a biochemical reaction. Biomolecules and the principles of enzyme kinetics and bioenergetics. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the reactants is large. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry.
From www.youtube.com
The equilibrium expression and the equilibrium constant (Keq) YouTube Enzymes Increase Keq Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. In this case, the reaction favors the. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. If keq is a small number (keq<1), it means that, at equilibrium, the concentration. Enzymes Increase Keq.
From www.bartleby.com
Figure 4.10 Enzymes, temperature, and pH. Each enzyme works best within Enzymes Increase Keq If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the reactants is large. Enzymes do not affect the thermodynamics of reactions. Biomolecules and the principles of enzyme kinetics and bioenergetics. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. For reversible reactions (as an example), the. Enzymes Increase Keq.
From ibiologia.com
Enzymes Functions Definition Classification Enzymes Increase Keq Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. In this case, the reaction favors the. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. These products then become involved in some other biological pathway that initiates certain functions. Enzymes Increase Keq.
From www.slideserve.com
PPT Introduction to ENZYMES PowerPoint Presentation, free download Enzymes Increase Keq The second quarter deals mainly with intermediary metabolism. Δg 0 and k eq remain the same. If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the reactants is large. In this case, the reaction favors the. Enzymes do not affect the thermodynamics of reactions. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the. Enzymes Increase Keq.
From studymind.co.uk
Enzymes Rates of Reaction (Alevel Biology) Study Mind Enzymes Increase Keq Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. The second quarter deals mainly with intermediary metabolism. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to. Enzymes Increase Keq.
From www.expii.com
Enzyme Inhibition — Overview & Types Expii Enzymes Increase Keq Biomolecules and the principles of enzyme kinetics and bioenergetics. This is known as the catalytic. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. Enzymes do not affect the thermodynamics of reactions. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. Enzymes are highly specific catalysts for biochemical reactions, with. Enzymes Increase Keq.
From studymind.co.uk
Enzymes Rates of Reaction (Alevel Biology) Study Mind Enzymes Increase Keq Enzymes do not affect the thermodynamics of reactions. If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the reactants is large. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms. Enzymes Increase Keq.
From www.researchgate.net
Backwards reaction rates of an enzyme directly upstream have a strong Enzymes Increase Keq Biomolecules and the principles of enzyme kinetics and bioenergetics. These products then become involved in some other biological pathway that initiates certain functions of the human body. This is known as the catalytic. The second quarter deals mainly with intermediary metabolism. If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the reactants is large.. Enzymes Increase Keq.
From boileddownbrewing.com
From Starch to Fermentable Sugars Enzymes in the Mash Enzymes Increase Keq Biomolecules and the principles of enzyme kinetics and bioenergetics. This is known as the catalytic. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. In this case, the reaction favors the. If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the reactants is large. Δg 0 and k eq remain. Enzymes Increase Keq.
From stock.adobe.com
Science infographic diagram show factors affecting enzyme activity Enzymes Increase Keq This is known as the catalytic. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. In short, enzymes do not change the equilibrium state of a biochemical reaction. Δg 0. Enzymes Increase Keq.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Enzymes Increase Keq Biomolecules and the principles of enzyme kinetics and bioenergetics. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. In short, enzymes do not change the equilibrium state of a biochemical reaction. In this case, the reaction favors the. Enzymes. Enzymes Increase Keq.
From www.linkedin.com
Soil Enzymes Enzymes Increase Keq Δg 0 and k eq remain the same. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. Biomolecules and the principles of enzyme kinetics and bioenergetics. If keq is a small number (keq<1), it means that, at equilibrium, the. Enzymes Increase Keq.
From jan.ucc.nau.edu
Enzymes and Reaction Rates Enzymes Increase Keq Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. Biomolecules and the principles of enzyme kinetics and bioenergetics. Δg 0 and k eq remain the same. Examining enzyme kinetics is. Enzymes Increase Keq.
From www.genome.gov
Enzyme Enzymes Increase Keq In this case, the reaction favors the. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. Biomolecules and the principles of enzyme kinetics and bioenergetics. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in. Enzymes Increase Keq.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Enzymes Increase Keq This is known as the catalytic. Δg 0 and k eq remain the same. Enzymes do not affect the thermodynamics of reactions. These products then become involved in some other biological pathway that initiates certain functions of the human body. In this case, the reaction favors the. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a. Enzymes Increase Keq.
From triyambak.org
For the enzymecatalyzed reaction S P, Keq = 5, which of the Enzymes Increase Keq In this case, the reaction favors the. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the. Enzymes Increase Keq.
From www.slideserve.com
PPT Equilibrium Expression (Keq) PowerPoint Presentation, free Enzymes Increase Keq This is known as the catalytic. If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the reactants is large. Δg 0 and k eq remain the same. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. In short, enzymes do not change the. Enzymes Increase Keq.
From philschatz.com
Chemical Reactions · Anatomy and Physiology Enzymes Increase Keq This is known as the catalytic. In short, enzymes do not change the equilibrium state of a biochemical reaction. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the reactants is large. Enzymes do not affect the thermodynamics of. Enzymes Increase Keq.
From www.researchgate.net
Schematic representation of the enzymes involved in the process of food Enzymes Increase Keq These products then become involved in some other biological pathway that initiates certain functions of the human body. The second quarter deals mainly with intermediary metabolism. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Enzymes do not affect. Enzymes Increase Keq.
From www.numerade.com
SOLVED Which of the following statements about enzyme catalyzed Enzymes Increase Keq For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. These products then become involved in some other biological. Enzymes Increase Keq.
From www.slideserve.com
PPT Outline of Enzymes PowerPoint Presentation, free download ID364722 Enzymes Increase Keq For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. These products then become involved in some other biological pathway. Enzymes Increase Keq.
From www.toppr.com
How do enzymes increase the rate of reaction? Enzymes Increase Keq These products then become involved in some other biological pathway that initiates certain functions of the human body. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. Δg 0 and. Enzymes Increase Keq.
From socratic.org
How do enzymes speed up the chemical reactions? Use activation energy Enzymes Increase Keq The second quarter deals mainly with intermediary metabolism. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant,. Enzymes Increase Keq.
From www.researchgate.net
5 Enzymes increase the rate of reaction Download Scientific Diagram Enzymes Increase Keq These products then become involved in some other biological pathway that initiates certain functions of the human body. Δg 0 and k eq remain the same. If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the reactants is large. The second quarter deals mainly with intermediary metabolism. The equilibrium constant of this reaction can. Enzymes Increase Keq.
From ecampusontario.pressbooks.pub
Protein binding & Recognition Chemical Basis of Enzyme Catalysis Enzymes Increase Keq For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. Enzymes do not affect the thermodynamics of reactions. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Δg 0 and k eq remain the same. These products then become involved in some other biological pathway that initiates certain functions. Enzymes Increase Keq.
From www.creative-enzymes.com
Catalytic Characteristics of Enzymes Creative Enzymes Enzymes Increase Keq Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. In this case, the reaction favors the. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or. Enzymes Increase Keq.
From slideplayer.com
Ch.24 Chemical Reactions & Enzymes ppt download Enzymes Increase Keq This is known as the catalytic. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. These products then become involved in some other biological pathway that initiates certain functions of the human body. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. In short, enzymes. Enzymes Increase Keq.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Enzymes Increase Keq In this case, the reaction favors the. The second quarter deals mainly with intermediary metabolism. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. This is known as the catalytic. If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the reactants is large.. Enzymes Increase Keq.
From www.youtube.com
Enzymes Part 2 DBT JRF PYQs Keq ∆G Specific activity Enzymes Increase Keq For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. Δg 0 and k eq remain the same. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. This is known as the. Enzymes Increase Keq.
From www.expii.com
Concentration (Enzyme Reaction Rates) — Effects & Role Expii Enzymes Increase Keq The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. These products then become involved in some other biological pathway that initiates certain functions of the human body. Δg 0 and k eq remain the same. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. The second. Enzymes Increase Keq.
From biology4ibdp.weebly.com
3.6 Enzymes BIOLOGY4IBDP Enzymes Increase Keq These products then become involved in some other biological pathway that initiates certain functions of the human body. The equilibrium constant of this reaction can be expressed in terms of reaction quotient at equilibrium, in terms of. Enzymes do not affect the thermodynamics of reactions. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for. Enzymes Increase Keq.
From www.scribd.com
Enzymes in Food Processing PDF Enzyme Food And Drink Enzymes Increase Keq Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. The second quarter deals mainly with intermediary metabolism. This is known as the catalytic. In this case, the reaction favors the. Enzymes do not affect the thermodynamics of reactions. If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the. Enzymes Increase Keq.
From www.bartleby.com
Answered Which of the following statement is… bartleby Enzymes Increase Keq This is known as the catalytic. Enzymes do not affect the thermodynamics of reactions. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. If keq is a small number (keq<1), it means that, at equilibrium, the concentration of the reactants is large. The equilibrium constant of this reaction can. Enzymes Increase Keq.
From www.chegg.com
Solved Question 1Which of the following statements about Enzymes Increase Keq In short, enzymes do not change the equilibrium state of a biochemical reaction. In this case, the reaction favors the. Enzymes do not affect the thermodynamics of reactions. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or. Enzymes Increase Keq.
From www.bartleby.com
Answered 10. Which of the following could… bartleby Enzymes Increase Keq Enzymes do not affect the thermodynamics of reactions. These products then become involved in some other biological pathway that initiates certain functions of the human body. In this case, the reaction favors the. In short, enzymes do not change the equilibrium state of a biochemical reaction. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in. Enzymes Increase Keq.