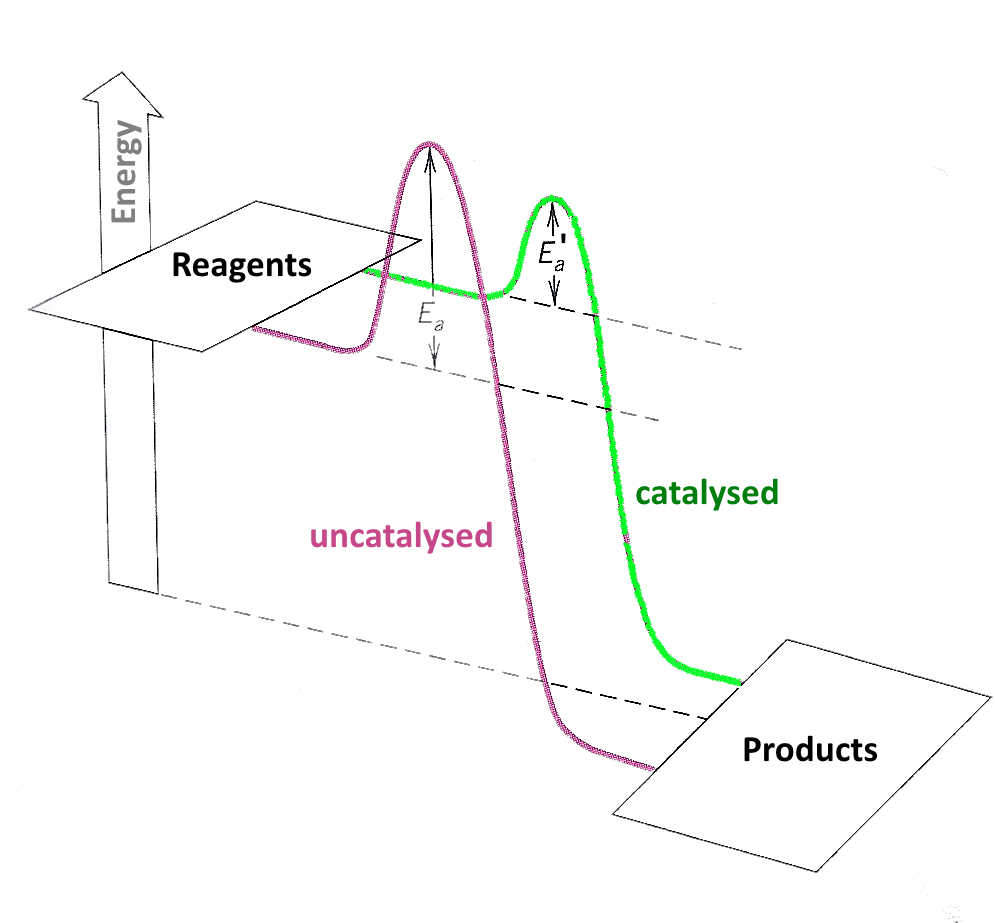
Catalyst Activation Energy Of A Reaction . A small quantity of catalyst should be able to affect the rate of reaction. catalysts increase the rate of reaction. List examples of catalysis in. Catalysts are not consumed by the. Enzymes are examples of catalysts. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; Catalysts can be homogenous (in the same. When a catalyst is used at 300 k, the rate increases one hundred fold. the activation energy of a reaction is 19.0 kj/mol. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. a catalyst lowers the activation energy of a chemical reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts are not consumed by the reaction.
from www.chim.lu
the activation energy of a reaction is 19.0 kj/mol. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; Catalysts are not consumed by the reaction. List examples of catalysis in. A small quantity of catalyst should be able to affect the rate of reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts increase the rate of reaction. Enzymes are examples of catalysts. Catalysts can be homogenous (in the same. a catalyst lowers the activation energy of a chemical reaction.
Activation energy and catalysis
Catalyst Activation Energy Of A Reaction A small quantity of catalyst should be able to affect the rate of reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst lowers the activation energy of a chemical reaction. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. catalysts increase the rate of reaction. A small quantity of catalyst should be able to affect the rate of reaction. Enzymes are examples of catalysts. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; Catalysts are not consumed by the reaction. the activation energy of a reaction is 19.0 kj/mol. List examples of catalysis in. When a catalyst is used at 300 k, the rate increases one hundred fold. Catalysts can be homogenous (in the same. Catalysts are not consumed by the.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Activation Energy Of A Reaction catalysts increase the rate of reaction. A small quantity of catalyst should be able to affect the rate of reaction. When a catalyst is used at 300 k, the rate increases one hundred fold. Catalysts are not consumed by the reaction. Enzymes are examples of catalysts. List examples of catalysis in. the activation energy of a reaction is. Catalyst Activation Energy Of A Reaction.
From saylordotorg.github.io
Catalysis Catalyst Activation Energy Of A Reaction a catalyst lowers the activation energy of a chemical reaction. the activation energy of a reaction is 19.0 kj/mol. Catalysts can be homogenous (in the same. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. explain the function of a catalyst in terms. Catalyst Activation Energy Of A Reaction.
From printablebillye75.z14.web.core.windows.net
Energy Diagram Chemistry Explained Catalyst Activation Energy Of A Reaction catalysts increase the rate of reaction. Enzymes are examples of catalysts. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; Catalysts are not consumed by the. Catalysts are not consumed by the reaction. List examples of catalysis in. Catalysts can be homogenous (in the same. in the case of a biological. Catalyst Activation Energy Of A Reaction.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Catalyst Activation Energy Of A Reaction in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. Catalysts are not consumed by the reaction. catalysts increase the rate of reaction. a catalyst lowers the activation energy of a chemical reaction. A small quantity of catalyst should be able to affect the rate. Catalyst Activation Energy Of A Reaction.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent Catalyst Activation Energy Of A Reaction explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. When a catalyst is used at 300 k, the rate increases one hundred fold. Enzymes are examples of catalysts. the. Catalyst Activation Energy Of A Reaction.
From www.youtube.com
How does a CATALYST work ? YouTube Catalyst Activation Energy Of A Reaction Catalysts can be homogenous (in the same. the activation energy of a reaction is 19.0 kj/mol. catalysts increase the rate of reaction. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. a catalyst lowers the activation energy of a chemical reaction. Catalysts are. Catalyst Activation Energy Of A Reaction.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Activation Energy Of A Reaction List examples of catalysis in. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; the activation energy of a reaction is 19.0 kj/mol. a catalyst lowers the activation energy of a chemical reaction. Enzymes are examples of catalysts. in the case of a biological reaction, when an enzyme (a form. Catalyst Activation Energy Of A Reaction.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Activation Energy Of A Reaction explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. A small quantity of catalyst should be able to affect the rate of reaction. List examples of catalysis in. Catalysts are. Catalyst Activation Energy Of A Reaction.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Catalyst Activation Energy Of A Reaction the activation energy of a reaction is 19.0 kj/mol. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Enzymes are examples of catalysts. Catalysts are not consumed by the. Catalysts are not consumed. Catalyst Activation Energy Of A Reaction.
From chemistry.stackexchange.com
thermodynamics Catalytic energy profiles (is this Wikipedia image Catalyst Activation Energy Of A Reaction A small quantity of catalyst should be able to affect the rate of reaction. the activation energy of a reaction is 19.0 kj/mol. List examples of catalysis in. catalysts increase the rate of reaction. Enzymes are examples of catalysts. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate,. Catalyst Activation Energy Of A Reaction.
From www.chim.lu
Activation energy and catalysis Catalyst Activation Energy Of A Reaction Catalysts are not consumed by the reaction. catalysts increase the rate of reaction. the activation energy of a reaction is 19.0 kj/mol. A small quantity of catalyst should be able to affect the rate of reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts are not. Catalyst Activation Energy Of A Reaction.
From circuiteracktiergotorg.z13.web.core.windows.net
How To Read Energy Diagrams Chemistry Catalyst Activation Energy Of A Reaction catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. When a catalyst is used at 300 k, the rate increases one hundred fold. Catalysts can be homogenous (in the same. catalysts increase the rate of reaction. the activation energy of a reaction is 19.0 kj/mol. in the. Catalyst Activation Energy Of A Reaction.
From sciencenotes.org
Factors That Affect Reaction Rate Chemical Catalyst Activation Energy Of A Reaction Enzymes are examples of catalysts. A small quantity of catalyst should be able to affect the rate of reaction. a catalyst lowers the activation energy of a chemical reaction. Catalysts are not consumed by the reaction. the activation energy of a reaction is 19.0 kj/mol. catalysts increase the rate of reaction. in the case of a. Catalyst Activation Energy Of A Reaction.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.9.3 MaxwellBoltzmann Distributions翰林国际教育 Catalyst Activation Energy Of A Reaction Catalysts are not consumed by the. Enzymes are examples of catalysts. a catalyst lowers the activation energy of a chemical reaction. When a catalyst is used at 300 k, the rate increases one hundred fold. Catalysts are not consumed by the reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower. Catalyst Activation Energy Of A Reaction.
From infinitylearn.com
A catalyst increases the rate of reaction by Sri Chaitanya Infinity Catalyst Activation Energy Of A Reaction catalysts increase the rate of reaction. A small quantity of catalyst should be able to affect the rate of reaction. Catalysts are not consumed by the. List examples of catalysis in. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Enzymes are examples of catalysts. the activation energy. Catalyst Activation Energy Of A Reaction.
From www.chegg.com
Solved The Diagram Shown Above Shows The Reaction Profile... Catalyst Activation Energy Of A Reaction Catalysts are not consumed by the reaction. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. catalysts increase the rate of reaction. Catalysts can be homogenous (in the same. A small quantity of catalyst should be able to affect the rate of reaction. Enzymes are. Catalyst Activation Energy Of A Reaction.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst Activation Energy Of A Reaction Enzymes are examples of catalysts. the activation energy of a reaction is 19.0 kj/mol. catalysts increase the rate of reaction. a catalyst lowers the activation energy of a chemical reaction. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; List examples of catalysis in. in the case of a. Catalyst Activation Energy Of A Reaction.
From www.kosmotime.com
Activation Energy The Secret to Getting Started and Getting Finished Catalyst Activation Energy Of A Reaction catalysts increase the rate of reaction. When a catalyst is used at 300 k, the rate increases one hundred fold. List examples of catalysis in. Catalysts are not consumed by the reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same. Catalysts. Catalyst Activation Energy Of A Reaction.
From www.shutterstock.com
3+ Hundred Concentrated Introduce RoyaltyFree Images, Stock Photos Catalyst Activation Energy Of A Reaction in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. Catalysts can be homogenous (in the same. the activation energy of a reaction is 19.0 kj/mol. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; Enzymes are examples of. Catalyst Activation Energy Of A Reaction.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Activation Energy Of A Reaction in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. a catalyst lowers the activation energy of a chemical reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Enzymes are examples of catalysts. List examples. Catalyst Activation Energy Of A Reaction.
From studyonline.blog
What Is Activation Energy? Definition and Examples Catalyst Activation Energy Of A Reaction a catalyst lowers the activation energy of a chemical reaction. A small quantity of catalyst should be able to affect the rate of reaction. Enzymes are examples of catalysts. When a catalyst is used at 300 k, the rate increases one hundred fold. the activation energy of a reaction is 19.0 kj/mol. catalysts increase the rate of. Catalyst Activation Energy Of A Reaction.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Activation Energy Of A Reaction catalysts increase the rate of reaction. a catalyst lowers the activation energy of a chemical reaction. Enzymes are examples of catalysts. Catalysts are not consumed by the. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. Catalysts can be homogenous (in the same. When. Catalyst Activation Energy Of A Reaction.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Catalyst Activation Energy Of A Reaction Enzymes are examples of catalysts. Catalysts are not consumed by the. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; When a catalyst is used at 300 k, the rate increases one hundred fold. the activation energy of a reaction is 19.0 kj/mol. catalysts affect the rate of a chemical reaction. Catalyst Activation Energy Of A Reaction.
From www.pnnl.gov
Catalysis PNNL Catalyst Activation Energy Of A Reaction the activation energy of a reaction is 19.0 kj/mol. Catalysts are not consumed by the. Catalysts are not consumed by the reaction. a catalyst lowers the activation energy of a chemical reaction. A small quantity of catalyst should be able to affect the rate of reaction. List examples of catalysis in. Enzymes are examples of catalysts. Catalysts can. Catalyst Activation Energy Of A Reaction.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalyst Activation Energy Of A Reaction When a catalyst is used at 300 k, the rate increases one hundred fold. catalysts increase the rate of reaction. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; a catalyst lowers the activation energy of a chemical reaction. A small quantity of catalyst should be able to affect the rate. Catalyst Activation Energy Of A Reaction.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Catalyst Activation Energy Of A Reaction Enzymes are examples of catalysts. Catalysts are not consumed by the reaction. catalysts increase the rate of reaction. When a catalyst is used at 300 k, the rate increases one hundred fold. a catalyst lowers the activation energy of a chemical reaction. Catalysts can be homogenous (in the same. List examples of catalysis in. Catalysts are not consumed. Catalyst Activation Energy Of A Reaction.
From www.doubtnut.com
X = energy of activation with catalyst, Y = energy of activation wit Catalyst Activation Energy Of A Reaction Enzymes are examples of catalysts. Catalysts are not consumed by the. Catalysts can be homogenous (in the same. List examples of catalysis in. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; a catalyst lowers the activation energy of a chemical reaction. catalysts increase the rate of reaction. Catalysts are not. Catalyst Activation Energy Of A Reaction.
From ar.inspiredpencil.com
Catalyst Examples For Kids Catalyst Activation Energy Of A Reaction catalysts increase the rate of reaction. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. Catalysts are not consumed by the reaction. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; Enzymes are examples of catalysts. When a. Catalyst Activation Energy Of A Reaction.
From www.cheric.org
Chemical Reaction (Reaction rate) Catalyst Activation Energy Of A Reaction a catalyst lowers the activation energy of a chemical reaction. Catalysts can be homogenous (in the same. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; Enzymes are examples of catalysts. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation. Catalyst Activation Energy Of A Reaction.
From as-bio-and-chem.blogspot.com
Bio+Chem Notes. ^^ Recapping Rates of Reaction Catalyst Activation Energy Of A Reaction Catalysts are not consumed by the reaction. Catalysts can be homogenous (in the same. Catalysts are not consumed by the. List examples of catalysis in. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams;. Catalyst Activation Energy Of A Reaction.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Catalyst Activation Energy Of A Reaction explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts are not consumed by the reaction. List examples of catalysis in. Catalysts are not consumed by the. the activation energy of a reaction. Catalyst Activation Energy Of A Reaction.
From schoolbag.info
A catalyst speeds up a reaction by providing the reactants with an Catalyst Activation Energy Of A Reaction the activation energy of a reaction is 19.0 kj/mol. A small quantity of catalyst should be able to affect the rate of reaction. List examples of catalysis in. Catalysts can be homogenous (in the same. Enzymes are examples of catalysts. catalysts increase the rate of reaction. Catalysts are not consumed by the. When a catalyst is used at. Catalyst Activation Energy Of A Reaction.
From www.vrogue.co
Activation Energy Definition Formula Diagram Examples vrogue.co Catalyst Activation Energy Of A Reaction Catalysts are not consumed by the reaction. catalysts increase the rate of reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. When a catalyst is used at 300 k, the rate increases one hundred fold. Enzymes are examples of catalysts. List examples of catalysis in. Catalysts can be. Catalyst Activation Energy Of A Reaction.
From asenoveo6schematic.z14.web.core.windows.net
How To Read Energy Diagrams Chemistry Catalyst Activation Energy Of A Reaction Catalysts are not consumed by the. Catalysts can be homogenous (in the same. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Enzymes are examples of catalysts. the activation energy of a reaction is 19.0 kj/mol. When a catalyst is used at 300 k, the rate increases one hundred. Catalyst Activation Energy Of A Reaction.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalyst Activation Energy Of A Reaction A small quantity of catalyst should be able to affect the rate of reaction. List examples of catalysis in. a catalyst lowers the activation energy of a chemical reaction. Catalysts are not consumed by the reaction. Catalysts are not consumed by the. catalysts increase the rate of reaction. Enzymes are examples of catalysts. explain the function of. Catalyst Activation Energy Of A Reaction.