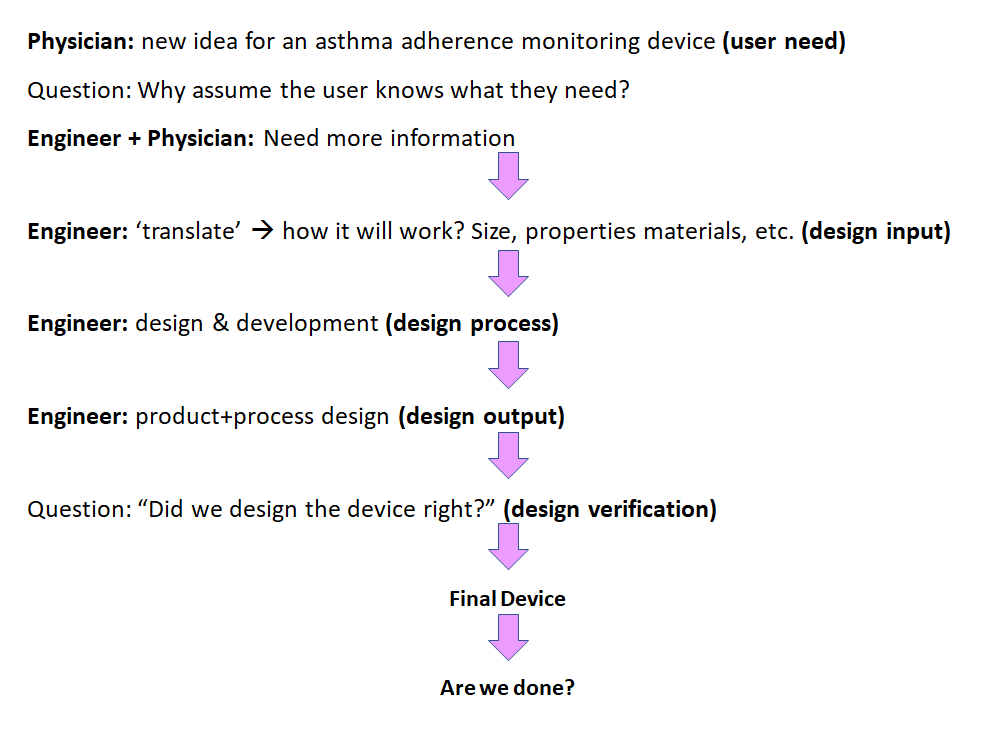
Medical Device User Needs Examples . The intended use, or the general purpose of the. the user needs document describes the intended use and indications for the device. Food and drug administration (fda). It must describe users’ needs in enough. In this guide, we’re looking at design inputs, user needs,. how can medical device designers bridge user needs and design requirements? this may seem obvious, but the needs of your users will define two critical definitions for your device: user experience (ux) for medical devices shifts the focus from solely upholding u.s. an example here would be a user needs and intended uses requirements document as well as a separate engineering requirements. Focus your uns on the benefits and the. Safe medical device act of 1990 authorized fda to add design controls to the current good.
from www.kolabtree.com
The intended use, or the general purpose of the. an example here would be a user needs and intended uses requirements document as well as a separate engineering requirements. It must describe users’ needs in enough. this may seem obvious, but the needs of your users will define two critical definitions for your device: Food and drug administration (fda). how can medical device designers bridge user needs and design requirements? Safe medical device act of 1990 authorized fda to add design controls to the current good. the user needs document describes the intended use and indications for the device. user experience (ux) for medical devices shifts the focus from solely upholding u.s. Focus your uns on the benefits and the.
Medical Device Design The Essential, StepbyStep Guide
Medical Device User Needs Examples the user needs document describes the intended use and indications for the device. In this guide, we’re looking at design inputs, user needs,. user experience (ux) for medical devices shifts the focus from solely upholding u.s. Focus your uns on the benefits and the. the user needs document describes the intended use and indications for the device. Safe medical device act of 1990 authorized fda to add design controls to the current good. The intended use, or the general purpose of the. an example here would be a user needs and intended uses requirements document as well as a separate engineering requirements. Food and drug administration (fda). how can medical device designers bridge user needs and design requirements? this may seem obvious, but the needs of your users will define two critical definitions for your device: It must describe users’ needs in enough.
From www.orielstat.com
Document Moved Medical Device User Needs Examples Food and drug administration (fda). The intended use, or the general purpose of the. this may seem obvious, but the needs of your users will define two critical definitions for your device: the user needs document describes the intended use and indications for the device. an example here would be a user needs and intended uses requirements. Medical Device User Needs Examples.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Medical Device User Needs Examples user experience (ux) for medical devices shifts the focus from solely upholding u.s. Focus your uns on the benefits and the. Food and drug administration (fda). an example here would be a user needs and intended uses requirements document as well as a separate engineering requirements. this may seem obvious, but the needs of your users will. Medical Device User Needs Examples.
From www.semanticscholar.org
[PDF] The Design of Medical Devices Semantic Scholar Medical Device User Needs Examples how can medical device designers bridge user needs and design requirements? The intended use, or the general purpose of the. Focus your uns on the benefits and the. the user needs document describes the intended use and indications for the device. Food and drug administration (fda). user experience (ux) for medical devices shifts the focus from solely. Medical Device User Needs Examples.
From simbex.com
How to Define Product Requirements for Medical Devices Medical Device User Needs Examples user experience (ux) for medical devices shifts the focus from solely upholding u.s. the user needs document describes the intended use and indications for the device. The intended use, or the general purpose of the. It must describe users’ needs in enough. an example here would be a user needs and intended uses requirements document as well. Medical Device User Needs Examples.
From www.greenlight.guru
10 Questions to Help Define User Needs Free Download Medical Device User Needs Examples the user needs document describes the intended use and indications for the device. this may seem obvious, but the needs of your users will define two critical definitions for your device: Focus your uns on the benefits and the. In this guide, we’re looking at design inputs, user needs,. how can medical device designers bridge user needs. Medical Device User Needs Examples.
From medicaldeviceacademy.com
Define medical device software verification and validation (V&V Medical Device User Needs Examples The intended use, or the general purpose of the. Food and drug administration (fda). user experience (ux) for medical devices shifts the focus from solely upholding u.s. It must describe users’ needs in enough. Safe medical device act of 1990 authorized fda to add design controls to the current good. how can medical device designers bridge user needs. Medical Device User Needs Examples.
From www.greenlight.guru
8 Questions That Define Your Medical Device User Needs Medical Device User Needs Examples user experience (ux) for medical devices shifts the focus from solely upholding u.s. how can medical device designers bridge user needs and design requirements? Safe medical device act of 1990 authorized fda to add design controls to the current good. this may seem obvious, but the needs of your users will define two critical definitions for your. Medical Device User Needs Examples.
From www.evidera.com
White Paper The Growing Need for RealWorld Evidence in Medical Medical Device User Needs Examples Safe medical device act of 1990 authorized fda to add design controls to the current good. this may seem obvious, but the needs of your users will define two critical definitions for your device: the user needs document describes the intended use and indications for the device. In this guide, we’re looking at design inputs, user needs,. Food. Medical Device User Needs Examples.
From www.semanticscholar.org
REQUIREMENTS CAPTURE FOR MEDICAL DEVICE DESIGN Semantic Scholar Medical Device User Needs Examples Food and drug administration (fda). this may seem obvious, but the needs of your users will define two critical definitions for your device: how can medical device designers bridge user needs and design requirements? In this guide, we’re looking at design inputs, user needs,. Focus your uns on the benefits and the. Safe medical device act of 1990. Medical Device User Needs Examples.
From operonstrategist.com
FDA Design Control The Ultimate Guide For Medical Device Companies Medical Device User Needs Examples how can medical device designers bridge user needs and design requirements? user experience (ux) for medical devices shifts the focus from solely upholding u.s. Safe medical device act of 1990 authorized fda to add design controls to the current good. The intended use, or the general purpose of the. the user needs document describes the intended use. Medical Device User Needs Examples.
From www.qualio.com
Ultimate guide to medical device design controls Medical Device User Needs Examples Food and drug administration (fda). Safe medical device act of 1990 authorized fda to add design controls to the current good. how can medical device designers bridge user needs and design requirements? this may seem obvious, but the needs of your users will define two critical definitions for your device: an example here would be a user. Medical Device User Needs Examples.
From globalstrategicsolutions.com
Medical Devices Archives Global Strategic Solutions Medical Device User Needs Examples user experience (ux) for medical devices shifts the focus from solely upholding u.s. Safe medical device act of 1990 authorized fda to add design controls to the current good. In this guide, we’re looking at design inputs, user needs,. Food and drug administration (fda). this may seem obvious, but the needs of your users will define two critical. Medical Device User Needs Examples.
From simbex.com
How to Define Product Requirements for Medical Devices Medical Device User Needs Examples the user needs document describes the intended use and indications for the device. In this guide, we’re looking at design inputs, user needs,. Safe medical device act of 1990 authorized fda to add design controls to the current good. The intended use, or the general purpose of the. Food and drug administration (fda). It must describe users’ needs in. Medical Device User Needs Examples.
From www.greenlight.guru
Ultimate Guide to Agile Design and Development for Medical Devices Medical Device User Needs Examples Food and drug administration (fda). In this guide, we’re looking at design inputs, user needs,. this may seem obvious, but the needs of your users will define two critical definitions for your device: the user needs document describes the intended use and indications for the device. Safe medical device act of 1990 authorized fda to add design controls. Medical Device User Needs Examples.
From www.greenlight.guru
8 Questions That Define Your Medical Device User Needs Medical Device User Needs Examples user experience (ux) for medical devices shifts the focus from solely upholding u.s. Focus your uns on the benefits and the. In this guide, we’re looking at design inputs, user needs,. this may seem obvious, but the needs of your users will define two critical definitions for your device: the user needs document describes the intended use. Medical Device User Needs Examples.
From www.idc.uk.com
Six Key Factors for Successful Medical Device Development Medical Device User Needs Examples Safe medical device act of 1990 authorized fda to add design controls to the current good. user experience (ux) for medical devices shifts the focus from solely upholding u.s. It must describe users’ needs in enough. Food and drug administration (fda). Focus your uns on the benefits and the. In this guide, we’re looking at design inputs, user needs,.. Medical Device User Needs Examples.
From www.youtube.com
Determining User Needs for Your Medical Device YouTube Medical Device User Needs Examples Food and drug administration (fda). The intended use, or the general purpose of the. this may seem obvious, but the needs of your users will define two critical definitions for your device: It must describe users’ needs in enough. Focus your uns on the benefits and the. user experience (ux) for medical devices shifts the focus from solely. Medical Device User Needs Examples.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device User Needs Examples Focus your uns on the benefits and the. The intended use, or the general purpose of the. Food and drug administration (fda). Safe medical device act of 1990 authorized fda to add design controls to the current good. how can medical device designers bridge user needs and design requirements? the user needs document describes the intended use and. Medical Device User Needs Examples.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Medical Device User Needs Examples an example here would be a user needs and intended uses requirements document as well as a separate engineering requirements. Safe medical device act of 1990 authorized fda to add design controls to the current good. Food and drug administration (fda). how can medical device designers bridge user needs and design requirements? In this guide, we’re looking at. Medical Device User Needs Examples.
From dribbble.com
Medical device user interface illustrations by Alan Lee on Dribbble Medical Device User Needs Examples Focus your uns on the benefits and the. Food and drug administration (fda). how can medical device designers bridge user needs and design requirements? an example here would be a user needs and intended uses requirements document as well as a separate engineering requirements. The intended use, or the general purpose of the. It must describe users’ needs. Medical Device User Needs Examples.
From www.youtube.com
User Needs and Design Inputs YouTube Medical Device User Needs Examples an example here would be a user needs and intended uses requirements document as well as a separate engineering requirements. Safe medical device act of 1990 authorized fda to add design controls to the current good. Food and drug administration (fda). the user needs document describes the intended use and indications for the device. The intended use, or. Medical Device User Needs Examples.
From www.youtube.com
Incorporating User Needs into Medical Device Product Development YouTube Medical Device User Needs Examples Safe medical device act of 1990 authorized fda to add design controls to the current good. Food and drug administration (fda). user experience (ux) for medical devices shifts the focus from solely upholding u.s. Focus your uns on the benefits and the. The intended use, or the general purpose of the. the user needs document describes the intended. Medical Device User Needs Examples.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device User Needs Examples how can medical device designers bridge user needs and design requirements? Safe medical device act of 1990 authorized fda to add design controls to the current good. the user needs document describes the intended use and indications for the device. an example here would be a user needs and intended uses requirements document as well as a. Medical Device User Needs Examples.
From old.sermitsiaq.ag
Medical Device Traceability Matrix Template Medical Device User Needs Examples Safe medical device act of 1990 authorized fda to add design controls to the current good. an example here would be a user needs and intended uses requirements document as well as a separate engineering requirements. this may seem obvious, but the needs of your users will define two critical definitions for your device: Food and drug administration. Medical Device User Needs Examples.
From www.greenlight.guru
Design Controls Need to Start With User Needs Medical Device User Needs Examples an example here would be a user needs and intended uses requirements document as well as a separate engineering requirements. Safe medical device act of 1990 authorized fda to add design controls to the current good. this may seem obvious, but the needs of your users will define two critical definitions for your device: how can medical. Medical Device User Needs Examples.
From br.pinterest.com
Healthcare has more individualized and user friendly on personal Medical Device User Needs Examples the user needs document describes the intended use and indications for the device. Safe medical device act of 1990 authorized fda to add design controls to the current good. In this guide, we’re looking at design inputs, user needs,. The intended use, or the general purpose of the. this may seem obvious, but the needs of your users. Medical Device User Needs Examples.
From www.greenlight.guru
Design Controls For Medical Device Companies [Guide] Medical Device User Needs Examples In this guide, we’re looking at design inputs, user needs,. the user needs document describes the intended use and indications for the device. user experience (ux) for medical devices shifts the focus from solely upholding u.s. Food and drug administration (fda). how can medical device designers bridge user needs and design requirements? The intended use, or the. Medical Device User Needs Examples.
From www.aplyon.com
Medical Device Product Performance Specification Procedure Medical Device User Needs Examples It must describe users’ needs in enough. Safe medical device act of 1990 authorized fda to add design controls to the current good. Food and drug administration (fda). Focus your uns on the benefits and the. this may seem obvious, but the needs of your users will define two critical definitions for your device: how can medical device. Medical Device User Needs Examples.
From medicaldevicehq.com
Medical device design control terminology Medical Device HQ 1 Medical Device User Needs Examples In this guide, we’re looking at design inputs, user needs,. The intended use, or the general purpose of the. how can medical device designers bridge user needs and design requirements? user experience (ux) for medical devices shifts the focus from solely upholding u.s. an example here would be a user needs and intended uses requirements document as. Medical Device User Needs Examples.
From www.presentationeze.com
Medical Device Instructions for Use Information & TrainingPresentationEZE Medical Device User Needs Examples an example here would be a user needs and intended uses requirements document as well as a separate engineering requirements. Food and drug administration (fda). Focus your uns on the benefits and the. Safe medical device act of 1990 authorized fda to add design controls to the current good. In this guide, we’re looking at design inputs, user needs,.. Medical Device User Needs Examples.
From innolitics.com
Design Inputs Best Practices, FAQs, and Examples Medical Device User Needs Examples In this guide, we’re looking at design inputs, user needs,. Focus your uns on the benefits and the. the user needs document describes the intended use and indications for the device. Food and drug administration (fda). this may seem obvious, but the needs of your users will define two critical definitions for your device: how can medical. Medical Device User Needs Examples.
From www.orielstat.com
PMSR_PSUR Oriel STAT A MATRIX Blog Medical Device User Needs Examples In this guide, we’re looking at design inputs, user needs,. Focus your uns on the benefits and the. It must describe users’ needs in enough. how can medical device designers bridge user needs and design requirements? The intended use, or the general purpose of the. an example here would be a user needs and intended uses requirements document. Medical Device User Needs Examples.
From www.orielstat.com
Medical Device Regulatory Training Requirements for Employees Medical Device User Needs Examples It must describe users’ needs in enough. this may seem obvious, but the needs of your users will define two critical definitions for your device: an example here would be a user needs and intended uses requirements document as well as a separate engineering requirements. Focus your uns on the benefits and the. Food and drug administration (fda).. Medical Device User Needs Examples.
From www.modernrequirements.com
Facilitate the Medical Device Design Controls Modern Requirements Medical Device User Needs Examples this may seem obvious, but the needs of your users will define two critical definitions for your device: how can medical device designers bridge user needs and design requirements? an example here would be a user needs and intended uses requirements document as well as a separate engineering requirements. user experience (ux) for medical devices shifts. Medical Device User Needs Examples.
From www.emmanuelbaccelli.org
Medical Device Design And Development Plan Template Medical Device User Needs Examples Food and drug administration (fda). It must describe users’ needs in enough. the user needs document describes the intended use and indications for the device. In this guide, we’re looking at design inputs, user needs,. how can medical device designers bridge user needs and design requirements? this may seem obvious, but the needs of your users will. Medical Device User Needs Examples.