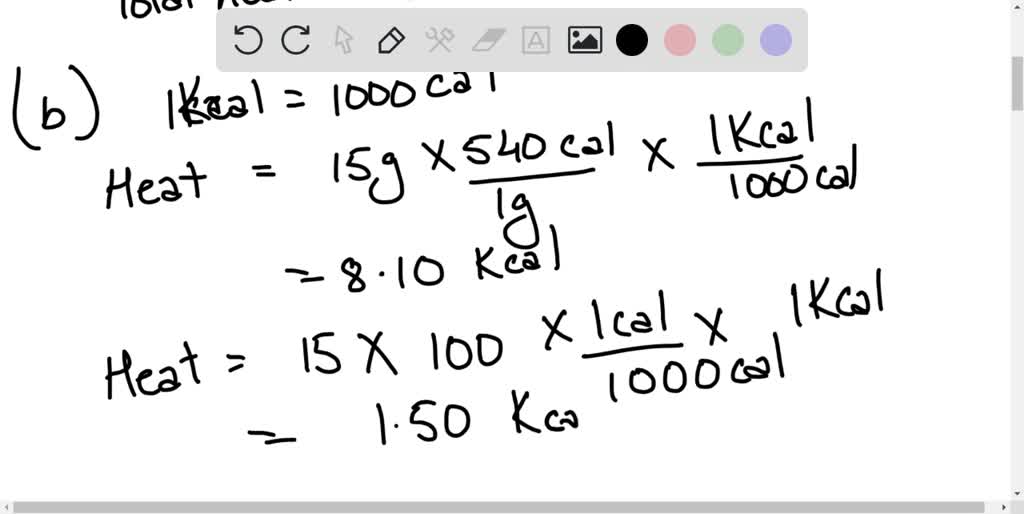Calorimetry Heat Of Fusion Formula . Additionally, it can find the. When we know how much movement of level a corresponds to how much transfer of heat), the calorimeter can be used to measure. Once the equipment has been calibrated (i.e. The calorimetry calculator can help you solve complex calorimetry problems. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. We now introduce two concepts useful in describing heat flow and temperature change. This involves measuring the temperature changes of. By drawing this chart before conducting a heat of fusion analysis, one can easily map out the required steps in completing the analysis. Experimentally, the heat of fusion can be determined through calorimetry. The heat lost by the warm object is equal to the heat gained by the cooler. It can analyze the heat exchange between up to 3 objects. To use calorimetric data to calculate enthalpy changes. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry.
from www.numerade.com
Additionally, it can find the. The heat lost by the warm object is equal to the heat gained by the cooler. To use calorimetric data to calculate enthalpy changes. Once the equipment has been calibrated (i.e. We now introduce two concepts useful in describing heat flow and temperature change. It can analyze the heat exchange between up to 3 objects. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. This involves measuring the temperature changes of. By drawing this chart before conducting a heat of fusion analysis, one can easily map out the required steps in completing the analysis.
Using the values for the heat of fusion, specific heat of water, and/or
Calorimetry Heat Of Fusion Formula Once the equipment has been calibrated (i.e. When we know how much movement of level a corresponds to how much transfer of heat), the calorimeter can be used to measure. Additionally, it can find the. The calorimetry calculator can help you solve complex calorimetry problems. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. It can analyze the heat exchange between up to 3 objects. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. By drawing this chart before conducting a heat of fusion analysis, one can easily map out the required steps in completing the analysis. To use calorimetric data to calculate enthalpy changes. We now introduce two concepts useful in describing heat flow and temperature change. Experimentally, the heat of fusion can be determined through calorimetry. Once the equipment has been calibrated (i.e. This involves measuring the temperature changes of. The heat lost by the warm object is equal to the heat gained by the cooler.
From www.tessshebaylo.com
Water Heating Equation Tessshebaylo Calorimetry Heat Of Fusion Formula One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. We now introduce two concepts useful in describing heat flow and temperature change. The heat lost by the warm object is equal to the heat gained by the cooler. When we know how much movement of level a. Calorimetry Heat Of Fusion Formula.
From studylib.net
Heat Capacity of Metals PreLab Calorimetry Heat Of Fusion Formula By drawing this chart before conducting a heat of fusion analysis, one can easily map out the required steps in completing the analysis. We now introduce two concepts useful in describing heat flow and temperature change. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. It can analyze the heat exchange between up to. Calorimetry Heat Of Fusion Formula.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Calorimetry Heat Of Fusion Formula It can analyze the heat exchange between up to 3 objects. Experimentally, the heat of fusion can be determined through calorimetry. Once the equipment has been calibrated (i.e. Additionally, it can find the. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. To use calorimetric data to calculate enthalpy changes. One technique we can. Calorimetry Heat Of Fusion Formula.
From www.youtube.com
Latent heat of fusion of ice YouTube Calorimetry Heat Of Fusion Formula We now introduce two concepts useful in describing heat flow and temperature change. To use calorimetric data to calculate enthalpy changes. Experimentally, the heat of fusion can be determined through calorimetry. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. This involves measuring the temperature changes of. It can analyze the heat exchange between. Calorimetry Heat Of Fusion Formula.
From louis.pressbooks.pub
Calorimetry (9.2) General Chemistry Calorimetry Heat Of Fusion Formula Once the equipment has been calibrated (i.e. It can analyze the heat exchange between up to 3 objects. To use calorimetric data to calculate enthalpy changes. We now introduce two concepts useful in describing heat flow and temperature change. This involves measuring the temperature changes of. One technique we can use to measure the amount of heat involved in a. Calorimetry Heat Of Fusion Formula.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Calorimetry Heat Of Fusion Formula This involves measuring the temperature changes of. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. We now introduce two concepts useful in describing heat flow and temperature change. The heat lost by the warm object is equal to the heat gained by the cooler. Experimentally, the. Calorimetry Heat Of Fusion Formula.
From slideplayer.com
STS Temperature and Heat ppt download Calorimetry Heat Of Fusion Formula Experimentally, the heat of fusion can be determined through calorimetry. Additionally, it can find the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. We now introduce two concepts useful in describing heat flow and temperature change. The equation for heat, q = m x #c_s# x. Calorimetry Heat Of Fusion Formula.
From lavelle.chem.ucla.edu
Difference CHEMISTRY COMMUNITY Calorimetry Heat Of Fusion Formula Experimentally, the heat of fusion can be determined through calorimetry. It can analyze the heat exchange between up to 3 objects. This involves measuring the temperature changes of. The heat lost by the warm object is equal to the heat gained by the cooler. Additionally, it can find the. The equation for heat, q = m x #c_s# x #deltat#. Calorimetry Heat Of Fusion Formula.
From www.youtube.com
CHEM 101 Calculating Enthalpy of Solution YouTube Calorimetry Heat Of Fusion Formula One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To use calorimetric data to calculate enthalpy changes. By drawing this chart before conducting a heat of fusion analysis, one can easily map out the required steps in completing the analysis. This involves measuring the temperature changes of.. Calorimetry Heat Of Fusion Formula.
From www.youtube.com
Calculating Heat of fusion YouTube Calorimetry Heat Of Fusion Formula Once the equipment has been calibrated (i.e. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. Experimentally, the heat of fusion can be determined through calorimetry. When we know how much movement of level a corresponds to how much transfer of heat), the calorimeter can be used to measure. To use calorimetric data to. Calorimetry Heat Of Fusion Formula.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Calorimetry Heat Of Fusion Formula When we know how much movement of level a corresponds to how much transfer of heat), the calorimeter can be used to measure. Additionally, it can find the. Experimentally, the heat of fusion can be determined through calorimetry. By drawing this chart before conducting a heat of fusion analysis, one can easily map out the required steps in completing the. Calorimetry Heat Of Fusion Formula.
From www.slideserve.com
PPT Heat of Fusion PowerPoint Presentation, free download ID2249917 Calorimetry Heat Of Fusion Formula It can analyze the heat exchange between up to 3 objects. When we know how much movement of level a corresponds to how much transfer of heat), the calorimeter can be used to measure. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. To use calorimetric data to calculate enthalpy changes. Additionally, it can. Calorimetry Heat Of Fusion Formula.
From www.slideshare.net
Energy ch 16 Calorimetry Heat Of Fusion Formula It can analyze the heat exchange between up to 3 objects. Experimentally, the heat of fusion can be determined through calorimetry. We now introduce two concepts useful in describing heat flow and temperature change. By drawing this chart before conducting a heat of fusion analysis, one can easily map out the required steps in completing the analysis. The heat lost. Calorimetry Heat Of Fusion Formula.
From ar.inspiredpencil.com
Heat And Fusion Definition Calorimetry Heat Of Fusion Formula One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Once the equipment has been calibrated (i.e. We now introduce two concepts useful in describing heat flow and temperature change. Additionally, it can find the. The calorimetry calculator can help you solve complex calorimetry problems. When we know. Calorimetry Heat Of Fusion Formula.
From laurennewsleach.blogspot.com
Latent Heat of Fusion of Ice Calorimetry Heat Of Fusion Formula The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. The heat lost by the warm object is equal to the heat gained by the cooler. By drawing this chart before conducting a heat of fusion analysis, one can easily map out the required steps in completing the analysis. Once the equipment has been calibrated. Calorimetry Heat Of Fusion Formula.
From ruairilucja.blogspot.com
24+ Calculating Heat Of Fusion RuairiLucja Calorimetry Heat Of Fusion Formula The calorimetry calculator can help you solve complex calorimetry problems. Once the equipment has been calibrated (i.e. This involves measuring the temperature changes of. The heat lost by the warm object is equal to the heat gained by the cooler. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. When we know how much. Calorimetry Heat Of Fusion Formula.
From www.tessshebaylo.com
Equation For Heat Energy Change Tessshebaylo Calorimetry Heat Of Fusion Formula The heat lost by the warm object is equal to the heat gained by the cooler. Experimentally, the heat of fusion can be determined through calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. By drawing this chart before conducting a heat of fusion analysis, one. Calorimetry Heat Of Fusion Formula.
From studylib.net
Heat Equation Calorimetry Heat Of Fusion Formula It can analyze the heat exchange between up to 3 objects. Once the equipment has been calibrated (i.e. By drawing this chart before conducting a heat of fusion analysis, one can easily map out the required steps in completing the analysis. To use calorimetric data to calculate enthalpy changes. This involves measuring the temperature changes of. Experimentally, the heat of. Calorimetry Heat Of Fusion Formula.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Calorimetry Heat Of Fusion Formula One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The calorimetry calculator can help you solve complex calorimetry problems. Once the equipment has been calibrated (i.e. Additionally, it can find the. Experimentally, the heat of fusion can be determined through calorimetry. When we know how much movement. Calorimetry Heat Of Fusion Formula.
From www.slideserve.com
PPT Heat of Reactions PowerPoint Presentation, free download ID5747139 Calorimetry Heat Of Fusion Formula By drawing this chart before conducting a heat of fusion analysis, one can easily map out the required steps in completing the analysis. Additionally, it can find the. Experimentally, the heat of fusion can be determined through calorimetry. The heat lost by the warm object is equal to the heat gained by the cooler. The equation for heat, q =. Calorimetry Heat Of Fusion Formula.
From proper-cooking.info
Coffee Cup Calorimeter Diagram Calorimetry Heat Of Fusion Formula The heat lost by the warm object is equal to the heat gained by the cooler. We now introduce two concepts useful in describing heat flow and temperature change. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. The calorimetry calculator can help you solve complex calorimetry problems. To use calorimetric data to calculate. Calorimetry Heat Of Fusion Formula.
From lloydanni.blogspot.com
30+ Calculating Heat Of Fusion LloydAnni Calorimetry Heat Of Fusion Formula We now introduce two concepts useful in describing heat flow and temperature change. When we know how much movement of level a corresponds to how much transfer of heat), the calorimeter can be used to measure. To use calorimetric data to calculate enthalpy changes. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. By. Calorimetry Heat Of Fusion Formula.
From www.numerade.com
Using the values for the heat of fusion, specific heat of water, and/or Calorimetry Heat Of Fusion Formula One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. We now introduce two concepts useful in describing heat flow and temperature change. It can analyze the heat exchange between up to 3 objects. By drawing this chart before conducting a heat of fusion analysis, one can easily. Calorimetry Heat Of Fusion Formula.
From ar.inspiredpencil.com
Heat Of Fusion Equation Calorimetry Heat Of Fusion Formula The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. Experimentally, the heat of fusion can be determined through calorimetry. When we know how much movement of level a corresponds to how much transfer of heat), the calorimeter can be used to measure. It can analyze the heat exchange between up to 3 objects. This. Calorimetry Heat Of Fusion Formula.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimetry Heat Of Fusion Formula We now introduce two concepts useful in describing heat flow and temperature change. Experimentally, the heat of fusion can be determined through calorimetry. Once the equipment has been calibrated (i.e. This involves measuring the temperature changes of. The heat lost by the warm object is equal to the heat gained by the cooler. By drawing this chart before conducting a. Calorimetry Heat Of Fusion Formula.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 Calorimetry Heat Of Fusion Formula One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The heat lost by the warm object is equal to the heat gained by the cooler. To use calorimetric data to calculate enthalpy changes. The equation for heat, q = m x #c_s# x #deltat# is used for. Calorimetry Heat Of Fusion Formula.
From www.mdpi.com
Liquids Free FullText Application of Solution Calorimetry to Calorimetry Heat Of Fusion Formula The calorimetry calculator can help you solve complex calorimetry problems. Additionally, it can find the. Once the equipment has been calibrated (i.e. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. This involves measuring the temperature changes of. We now introduce two concepts useful in describing heat flow and temperature change. It can analyze. Calorimetry Heat Of Fusion Formula.
From study.com
Heat of Fusion Definition, Equation & Examples Video & Lesson Calorimetry Heat Of Fusion Formula We now introduce two concepts useful in describing heat flow and temperature change. Experimentally, the heat of fusion can be determined through calorimetry. This involves measuring the temperature changes of. It can analyze the heat exchange between up to 3 objects. The heat lost by the warm object is equal to the heat gained by the cooler. One technique we. Calorimetry Heat Of Fusion Formula.
From davida.davivienda.com
Heat Of Fusion And Vaporization Worksheet Printable Word Searches Calorimetry Heat Of Fusion Formula The heat lost by the warm object is equal to the heat gained by the cooler. Experimentally, the heat of fusion can be determined through calorimetry. Additionally, it can find the. By drawing this chart before conducting a heat of fusion analysis, one can easily map out the required steps in completing the analysis. One technique we can use to. Calorimetry Heat Of Fusion Formula.
From www.slideserve.com
PPT AP Thermochemistry AP Thermodynamics PowerPoint Presentation Calorimetry Heat Of Fusion Formula Once the equipment has been calibrated (i.e. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. It can analyze the heat exchange between up to 3 objects. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. We now introduce two concepts. Calorimetry Heat Of Fusion Formula.
From www.youtube.com
GCSE Physics All Exam Boards Thermal Physics Specific Heat Calorimetry Heat Of Fusion Formula To use calorimetric data to calculate enthalpy changes. The heat lost by the warm object is equal to the heat gained by the cooler. Experimentally, the heat of fusion can be determined through calorimetry. This involves measuring the temperature changes of. Additionally, it can find the. By drawing this chart before conducting a heat of fusion analysis, one can easily. Calorimetry Heat Of Fusion Formula.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimetry Heat Of Fusion Formula One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It can analyze the heat exchange between up to 3 objects. The heat lost by the warm object is equal to the heat gained by the cooler. Once the equipment has been calibrated (i.e. This involves measuring the. Calorimetry Heat Of Fusion Formula.
From www.slideserve.com
PPT Purpose of the Experiment PowerPoint Presentation, free download Calorimetry Heat Of Fusion Formula It can analyze the heat exchange between up to 3 objects. The calorimetry calculator can help you solve complex calorimetry problems. Once the equipment has been calibrated (i.e. We now introduce two concepts useful in describing heat flow and temperature change. Additionally, it can find the. By drawing this chart before conducting a heat of fusion analysis, one can easily. Calorimetry Heat Of Fusion Formula.
From www.youtube.com
Heating Curve and Cooling Curve of Water Enthalpy of Fusion Calorimetry Heat Of Fusion Formula The calorimetry calculator can help you solve complex calorimetry problems. To use calorimetric data to calculate enthalpy changes. By drawing this chart before conducting a heat of fusion analysis, one can easily map out the required steps in completing the analysis. Additionally, it can find the. This involves measuring the temperature changes of. Once the equipment has been calibrated (i.e.. Calorimetry Heat Of Fusion Formula.
From fr.thptnganamst.edu.vn
Découvrir 160+ imagen enthalpie de fusion formule fr.thptnganamst.edu.vn Calorimetry Heat Of Fusion Formula The calorimetry calculator can help you solve complex calorimetry problems. Additionally, it can find the. We now introduce two concepts useful in describing heat flow and temperature change. When we know how much movement of level a corresponds to how much transfer of heat), the calorimeter can be used to measure. It can analyze the heat exchange between up to. Calorimetry Heat Of Fusion Formula.