Microbial Testing Limits . Personnel should be made aware of: The 1st set of criteria. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. Such products should include tests and limits for microbial content in both the batch release and expiry specifications.
from www.slideshare.net
2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Such products should include tests and limits for microbial content in both the batch release and expiry specifications. Personnel should be made aware of: This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. The 1st set of criteria. This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality.
Microbial limit tests I.P By Dr.P.Srinivas Jangaon institute of pharm…
Microbial Testing Limits 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. The 1st set of criteria. Such products should include tests and limits for microbial content in both the batch release and expiry specifications. Personnel should be made aware of: This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel.
From microbiologyguideritesh.com
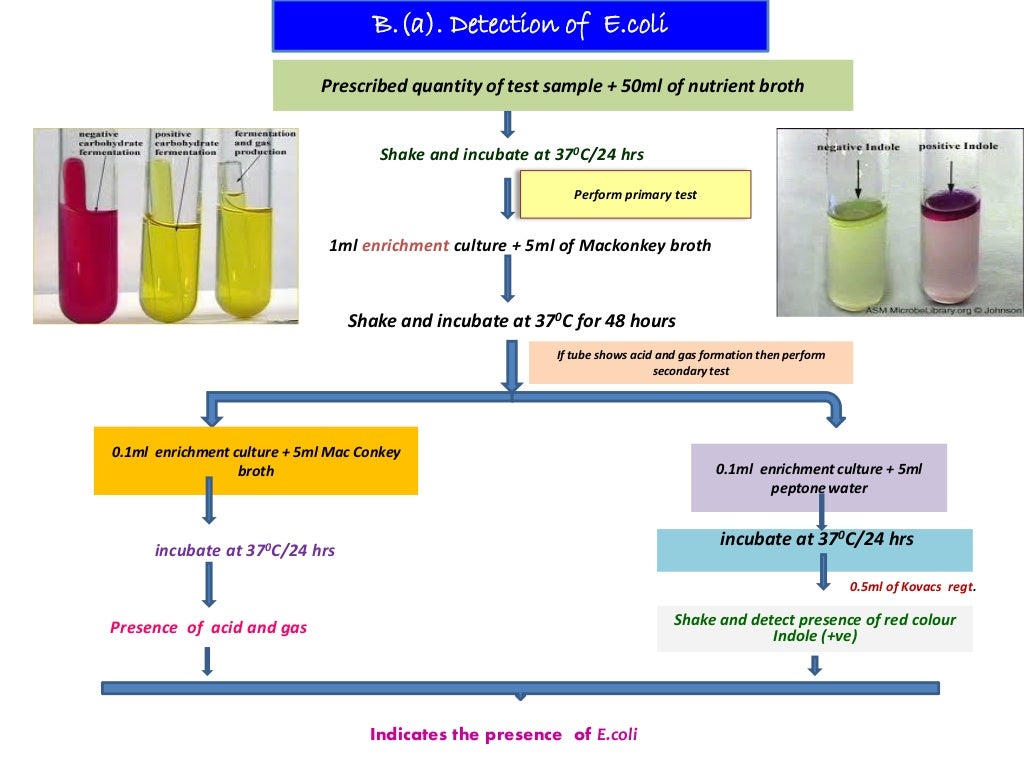
Flow Chart of Microbial Limit Test Microbiology Guidelines Microbial Testing Limits Personnel should be made aware of: 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. The 1st set of criteria. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; Such products should. Microbial Testing Limits.
From microbe-investigations.com
USP 61 Testing for Microbial Enumeration Microbe Investigations Microbial Testing Limits The 1st set of criteria. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. Such products should include tests and limits for microbial content in both the batch release and expiry specifications. This general chapter presents 2 sets of recommended. Microbial Testing Limits.
From www.itclabs.com
Microbial Contamination Testing at ITC Lab Assures Quality& Safety Microbial Testing Limits Personnel should be made aware of: Such products should include tests and limits for microbial content in both the batch release and expiry specifications. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. This chapter provides tests for the estimation of the number of. Microbial Testing Limits.
From www.slideshare.net
Microbial Limit Test An Over view Microbial Testing Limits Personnel should be made aware of: This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. Such products should include tests and limits for microbial content in both the batch release and expiry specifications. 2.1.5 access to the microbiological laboratory. Microbial Testing Limits.
From www.slideserve.com
PPT Practical aspects of Microbiological Testing and handling of OOS Microbial Testing Limits Personnel should be made aware of: The 1st set of criteria. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. Such products should include tests and limits for microbial content in both the batch release and expiry specifications. This general. Microbial Testing Limits.
From www.slideshare.net
Microbial limit test Microbial Testing Limits This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; Such products should include tests and limits for microbial content in both the batch release and expiry specifications. This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. Personnel should be made aware of: The 1st set of criteria. 2.1.5. Microbial Testing Limits.
From www.slideshare.net
Microbial limit tests I.P By Dr.P.Srinivas Jangaon institute of pharm… Microbial Testing Limits This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. The 1st set of criteria. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. 2.1.5 access to the microbiological laboratory should be. Microbial Testing Limits.
From cptclabs.com
Reliable Microbial Limits Test CPT Labs Microbial Testing Limits This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Personnel should be made aware of: This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. The 1st set of criteria. Such products should include tests and limits. Microbial Testing Limits.
From www.slideshare.net
Simple way to understand Microbial Limit Test Microbial Testing Limits Such products should include tests and limits for microbial content in both the batch release and expiry specifications. Personnel should be made aware of: The 1st set of criteria. This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. 2.1.5. Microbial Testing Limits.
From pharmablog.in
Microbial Limit Test for Raw Materials SOP PharmaBlog Microbial Testing Limits This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. The 1st set of criteria. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Such products should include tests and limits for microbial content in both the. Microbial Testing Limits.
From www.researchgate.net
Microbial limits for wastewater reuse in different countries Download Microbial Testing Limits Personnel should be made aware of: 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. The 1st set of criteria. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; Such products should include tests and limits. Microbial Testing Limits.
From pharmastate.academy
SOP for Microbial Limit Test to Estimate Viable Aerobic Micro Organisms Microbial Testing Limits This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; Personnel should be made aware of: Such products should include tests and limits for microbial content in both the batch release and expiry specifications. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. The 1st. Microbial Testing Limits.
From www.scribd.com
Microbial Limit Test Validation Protocol PDF Growth Medium Microbial Testing Limits Personnel should be made aware of: The 1st set of criteria. This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This chapter includes discussions on (1) the. Microbial Testing Limits.
From www.differencebetween.net
Difference Between Bioburden and Microbial Limit Test Difference Between Microbial Testing Limits 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; Personnel should be made aware of: This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. This general chapter presents 2 sets of recommended. Microbial Testing Limits.
From www.slideshare.net
Microbial limit test Microbial Testing Limits 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. Such products should include tests and limits for microbial content in both the batch release and expiry specifications.. Microbial Testing Limits.
From www.researchgate.net
microbial limits for botanical ingredients. Download Table Microbial Testing Limits This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; Personnel should be made aware of: Such products should include tests and limits for microbial content in both the batch release and expiry specifications. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This chapter provides tests for the estimation. Microbial Testing Limits.
From microbiologyguideritesh.com
Flow Chart of Microbial Limit Test Microbiology Guidelines Microbial Testing Limits Such products should include tests and limits for microbial content in both the batch release and expiry specifications. The 1st set of criteria. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This general chapter presents 2 sets of recommended. Microbial Testing Limits.
From www.youtube.com
Do you know the procedure of Microbial Limit Test? Microbial Analysis Microbial Testing Limits 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Personnel should be made aware of: This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; The 1st set of criteria. This chapter provides tests for the estimation. Microbial Testing Limits.
From www.researchgate.net
Existing microbiological limits and guidelines for Vibrio... Download Microbial Testing Limits This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. Such products should include tests and limits for microbial content in both the batch release and expiry specifications. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Personnel should be made aware of: The 1st set of criteria. This chapter. Microbial Testing Limits.
From bdn.go.th
10.2 MICROBIAL LIMIT TESTS ตำรายาของประเทศไทย กรมวิทยาศาสตร์การแพทย์ Microbial Testing Limits Personnel should be made aware of: 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. The 1st set of criteria. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; Such products should. Microbial Testing Limits.
From www.gbu-presnenskij.ru
Usp Dietary Supplements Microbial Limits Great Offers www.gbu Microbial Testing Limits This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. Such products should include tests and limits for microbial content in both the batch release and expiry specifications. The 1st set of criteria. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; This chapter provides tests for the estimation. Microbial Testing Limits.
From www.researchgate.net
Microbial limit test for formulations A to E. (Nil No growth of Microbial Testing Limits This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Such products should include tests and limits for microbial content in both the batch release and expiry specifications. Personnel should be made aware of: This chapter includes discussions on (1) the. Microbial Testing Limits.
From www.shutterstock.com
Lactobacillus Plantarum Microbial Limit Test Results Stock Photo (Edit Microbial Testing Limits 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Such products should include tests and limits for microbial content in both the batch release and expiry specifications. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; Personnel should be made aware of: This chapter provides tests for the estimation. Microbial Testing Limits.
From pacificbiolabs.com
Microbial Limits Test Pacific BioLabs Microbial Testing Limits The 1st set of criteria. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; Personnel should be made aware of: This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. Such products should include tests and limits for microbial content in both the batch release and expiry specifications. This. Microbial Testing Limits.
From pharmablog.in
Microbial Limit Test For Primary Packaging Materials SOP PharmaBlog Microbial Testing Limits This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; The 1st set of criteria. Such products should include tests and limits for microbial content in both the batch release and expiry specifications. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Personnel should be made aware of: This chapter. Microbial Testing Limits.
From www.differencebetween.net
Difference Between Bioburden and Microbial Limit Test Difference Between Microbial Testing Limits This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. Personnel should be made aware of: Such products should include tests and limits for microbial content in both the batch release and expiry specifications. The 1st set of criteria. This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. 2.1.5. Microbial Testing Limits.
From eduinput.com
What is the Difference Between Bioburden and Microbial Limit Test? Microbial Testing Limits This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; Personnel should be made aware of: The 1st set of criteria. This chapter provides tests for the estimation. Microbial Testing Limits.
From www.cirs-group.com
Microbiological Testing Microbiological Testing Medical Devices Microbial Testing Limits Such products should include tests and limits for microbial content in both the batch release and expiry specifications. Personnel should be made aware of: The 1st set of criteria. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. This chapter provides tests for the. Microbial Testing Limits.
From microbiologyguideritesh.com
Fundamentals of Microbial Limit Test 2023 Microbial Testing Limits Such products should include tests and limits for microbial content in both the batch release and expiry specifications. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. Personnel should be made aware of: This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. 2.1.5 access to the microbiological laboratory. Microbial Testing Limits.
From www.slideshare.net
Microbial limit tests I.P By Dr.P.Srinivas Jangaon institute of pharm… Microbial Testing Limits This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. Such products should include tests and limits for microbial content in both the batch release and expiry specifications. The 1st set of criteria. Personnel should be made aware of: This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; 2.1.5. Microbial Testing Limits.
From www.studocu.com
Microbial limit test MLT (Microbial Limit Test) Validation Learn how Microbial Testing Limits 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; Personnel should be made aware of: Such products should include tests and limits for microbial content in both the batch release and expiry specifications. This general chapter presents 2 sets of. Microbial Testing Limits.
From www.slideshare.net
Microbial limit tests I.P By Dr.P.Srinivas Jangaon institute of pharm… Microbial Testing Limits Such products should include tests and limits for microbial content in both the batch release and expiry specifications. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. The 1st set of criteria. This general chapter presents 2 sets of recommended. Microbial Testing Limits.
From www.slideshare.net
Microbial limit tests I.P By Dr.P.Srinivas Jangaon institute of pharm… Microbial Testing Limits Such products should include tests and limits for microbial content in both the batch release and expiry specifications. Personnel should be made aware of: This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. This chapter includes discussions on (1) the classification of a clean. Microbial Testing Limits.
From apnapharmaguru.com
Difference Between Bioburden Test and Microbiological Limit Test an Microbial Testing Limits This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. Personnel should be made aware of: Such products should include tests and limits for microbial content in both the batch release and expiry specifications. This general chapter presents 2 sets of recommended acceptance criteria for microbiological quality. This chapter includes discussions on (1). Microbial Testing Limits.
From www.slideshare.net
Microbial limit test Microbial Testing Limits The 1st set of criteria. This chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for. Such products should include tests and limits for microbial content in both the batch release and expiry specifications. This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; Personnel should be. Microbial Testing Limits.