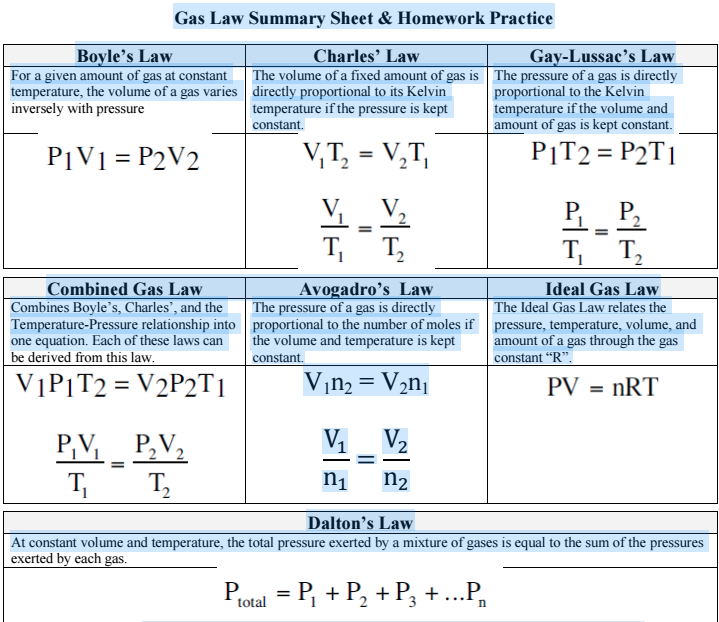
Chemistry Gas Laws Cheat Sheet . The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. The gas with the lower molar mass will be the. After the rates of diffusion/effusion for two gases are determined,. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas law reduces. Calculate the total pressure in the. 1 = mole fraction of gas. The most energetic of the three states of matter, gases, has several laws that describe behavior. When a gas is collected over water also in a mixture, the partial pressure of gas. The kinetic molecular theory attempts to explain gas behavior.
from scienceducatio.weebly.com
The gas with the lower molar mass will be the. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. When a gas is collected over water also in a mixture, the partial pressure of gas. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas law reduces. Calculate the total pressure in the. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. 1 = mole fraction of gas. After the rates of diffusion/effusion for two gases are determined,. The most energetic of the three states of matter, gases, has several laws that describe behavior. The kinetic molecular theory attempts to explain gas behavior.
Gas Laws Environmental Chemistry
Chemistry Gas Laws Cheat Sheet If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. 1 = mole fraction of gas. The kinetic molecular theory attempts to explain gas behavior. The gas with the lower molar mass will be the. When a gas is collected over water also in a mixture, the partial pressure of gas. The most energetic of the three states of matter, gases, has several laws that describe behavior. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas law reduces. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. Calculate the total pressure in the. After the rates of diffusion/effusion for two gases are determined,.
From www.studocu.com
Gas Laws Cheat Sheet 2012 Gas Laws Cheat Sheet STP is 1 atm and 0 C K = 273 C (Change ALL Chemistry Gas Laws Cheat Sheet The most energetic of the three states of matter, gases, has several laws that describe behavior. After the rates of diffusion/effusion for two gases are determined,. 1 = mole fraction of gas. The gas with the lower molar mass will be the. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml,. Chemistry Gas Laws Cheat Sheet.
From rapidlearningcenter.com
College Chemistry Gas Laws Chemistry Gas Laws Cheat Sheet The kinetic molecular theory attempts to explain gas behavior. The gas with the lower molar mass will be the. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas law reduces. After the rates of diffusion/effusion for two gases are determined,. The most energetic of the three states. Chemistry Gas Laws Cheat Sheet.
From www.youtube.com
Gas Law Formulas and Equations College Chemistry Study Guide YouTube Chemistry Gas Laws Cheat Sheet The most energetic of the three states of matter, gases, has several laws that describe behavior. 1 = mole fraction of gas. After the rates of diffusion/effusion for two gases are determined,. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas law reduces. The kinetic molecular theory. Chemistry Gas Laws Cheat Sheet.
From mungfali.com
Gas Laws Formula Sheet Chemistry Gas Laws Cheat Sheet 1 = mole fraction of gas. After the rates of diffusion/effusion for two gases are determined,. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The kinetic molecular theory attempts to explain gas behavior. Calculate the total pressure in the. The gas with the lower molar mass will be the. If a gas. Chemistry Gas Laws Cheat Sheet.
From docs.google.com
Gas Laws cheat sheet.docx Google Docs Chemistry Gas Laws Cheat Sheet The most energetic of the three states of matter, gases, has several laws that describe behavior. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The gas with the lower molar mass will be the. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant. Chemistry Gas Laws Cheat Sheet.
From www.studocu.com
Gas Laws practice sheet Pat constant constant VaT "Pressure is inversely "volume IS Studocu Chemistry Gas Laws Cheat Sheet 1 = mole fraction of gas. The most energetic of the three states of matter, gases, has several laws that describe behavior. Calculate the total pressure in the. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the. Chemistry Gas Laws Cheat Sheet.
From www.slideshare.net
Gases cheat sheet Chemistry Gas Laws Cheat Sheet The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. 1 = mole fraction of gas. The kinetic molecular theory attempts to explain gas behavior. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. When a. Chemistry Gas Laws Cheat Sheet.
From www.pearson.com
Chemistry Gas Laws Channels for Pearson+ Chemistry Gas Laws Cheat Sheet After the rates of diffusion/effusion for two gases are determined,. The gas with the lower molar mass will be the. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas law reduces. Calculate the total pressure in the. The kinetic molecular theory attempts to explain gas behavior. When. Chemistry Gas Laws Cheat Sheet.
From studylib.net
Gas Laws Cheat Sheet 2012 Chemistry Gas Laws Cheat Sheet Calculate the total pressure in the. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. 1 = mole fraction of gas. When a gas is collected. Chemistry Gas Laws Cheat Sheet.
From printablelistgather.z4.web.core.windows.net
Gas Law Formulas Sheet Chemistry Gas Laws Cheat Sheet The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas law reduces. After the rates of diffusion/effusion for two gases are determined,. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container. Chemistry Gas Laws Cheat Sheet.
From docest.com
Gas Laws Cheat Sheet Docest Chemistry Gas Laws Cheat Sheet 1 = mole fraction of gas. The kinetic molecular theory attempts to explain gas behavior. The most energetic of the three states of matter, gases, has several laws that describe behavior. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The ideal gas law relates the pressure, temperature, volume and moles of a. Chemistry Gas Laws Cheat Sheet.
From www.studocu.com
Gas Laws Cheat Sheet 2013 Perfect for notes Gas Laws Cheat Sheet STP is 1 atm and 0 C K = 273 Chemistry Gas Laws Cheat Sheet The gas with the lower molar mass will be the. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas law reduces. The kinetic molecular theory attempts to explain gas behavior. When a gas is collected over water also in a mixture, the partial pressure of gas. After. Chemistry Gas Laws Cheat Sheet.
From www.studypool.com
SOLUTION Study Guide Gas Laws Studypool Chemistry Gas Laws Cheat Sheet The gas with the lower molar mass will be the. 1 = mole fraction of gas. Calculate the total pressure in the. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. The kinetic molecular theory attempts to explain gas behavior. The ideal. Chemistry Gas Laws Cheat Sheet.
From www.slideshare.net
Gas lawscheat sheet Chemistry Gas Laws Cheat Sheet After the rates of diffusion/effusion for two gases are determined,. The most energetic of the three states of matter, gases, has several laws that describe behavior. Calculate the total pressure in the. 1 = mole fraction of gas. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas. Chemistry Gas Laws Cheat Sheet.
From www.scribd.com
Gas Laws Worksheet 2 Boyles Charles and Combined Gases Pressure Chemistry Gas Laws Cheat Sheet If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. When a gas is collected over water also in a mixture, the partial pressure of gas. After the rates of diffusion/effusion for two gases are determined,. The kinetic molecular theory attempts to explain. Chemistry Gas Laws Cheat Sheet.
From mungfali.com
Gas Laws Cheat Sheet Chemistry Gas Laws Cheat Sheet When a gas is collected over water also in a mixture, the partial pressure of gas. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas law reduces. The kinetic molecular theory attempts to explain gas behavior. The most energetic of the three states of matter, gases, has. Chemistry Gas Laws Cheat Sheet.
From www.studypool.com
SOLUTION Gas laws cheat sheet Studypool Chemistry Gas Laws Cheat Sheet The most energetic of the three states of matter, gases, has several laws that describe behavior. 1 = mole fraction of gas. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. After the rates of diffusion/effusion for two gases are determined,. The ideal gas law relates the pressure, temperature, volume and moles of. Chemistry Gas Laws Cheat Sheet.
From www.studocu.com
Gas laws cheat sheet Gas Laws Cheat Sheet STP is 1 atm and 0 C K = 273 + C (Change ALL temp to Chemistry Gas Laws Cheat Sheet The gas with the lower molar mass will be the. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. After the rates of diffusion/effusion for two gases are determined,. The kinetic molecular theory attempts to explain gas behavior. The three fundamental gas. Chemistry Gas Laws Cheat Sheet.
From www.chemistry24.com
High School Chemistry Gas Laws Chemistry Gas Laws Cheat Sheet The most energetic of the three states of matter, gases, has several laws that describe behavior. When a gas is collected over water also in a mixture, the partial pressure of gas. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. After. Chemistry Gas Laws Cheat Sheet.
From www.studypool.com
SOLUTION Gas laws key worksheet with answer Studypool Chemistry Gas Laws Cheat Sheet The most energetic of the three states of matter, gases, has several laws that describe behavior. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas. Chemistry Gas Laws Cheat Sheet.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 Chemistry Gas Laws Cheat Sheet The gas with the lower molar mass will be the. The kinetic molecular theory attempts to explain gas behavior. The most energetic of the three states of matter, gases, has several laws that describe behavior. 1 = mole fraction of gas. Calculate the total pressure in the. When a gas is collected over water also in a mixture, the partial. Chemistry Gas Laws Cheat Sheet.
From rapidlearningcenter.com
College Chemistry Gas Laws Chemistry Gas Laws Cheat Sheet When a gas is collected over water also in a mixture, the partial pressure of gas. Calculate the total pressure in the. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new. Chemistry Gas Laws Cheat Sheet.
From in.pinterest.com
This sheet gives the formulas for the Gas Laws. MA.912.HSAAPR.C [Cluster Statement] Use Chemistry Gas Laws Cheat Sheet After the rates of diffusion/effusion for two gases are determined,. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. When a gas is collected over water also in a mixture, the partial pressure of gas. The three fundamental gas laws discover the. Chemistry Gas Laws Cheat Sheet.
From www.homeworkminutes.com
What are the Different Gas Laws in Chemistry Chemistry Gas Laws Cheat Sheet If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. The gas with the lower molar mass will be the. Calculate the total pressure in the. The most energetic of the three states of matter, gases, has several laws that describe behavior. The. Chemistry Gas Laws Cheat Sheet.
From www.scribd.com
Gas Laws Cheat Sheet PDF Chemistry Gas Laws Cheat Sheet The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas law reduces. Calculate the total pressure in the. The most energetic of the three states of matter, gases, has several laws that describe behavior. The kinetic molecular theory attempts to explain gas behavior. When a gas is collected. Chemistry Gas Laws Cheat Sheet.
From www.studypool.com
SOLUTION Study Guide Gas Laws Studypool Chemistry Gas Laws Cheat Sheet The most energetic of the three states of matter, gases, has several laws that describe behavior. When a gas is collected over water also in a mixture, the partial pressure of gas. 1 = mole fraction of gas. After the rates of diffusion/effusion for two gases are determined,. The kinetic molecular theory attempts to explain gas behavior. The ideal gas. Chemistry Gas Laws Cheat Sheet.
From www.studocu.com
GC1 HO1 Gas Laws Cheat Sheet GAS LAWS CHEAT SHEET Gas Variables and Common Units Pressure Chemistry Gas Laws Cheat Sheet The most energetic of the three states of matter, gases, has several laws that describe behavior. 1 = mole fraction of gas. The gas with the lower molar mass will be the. When a gas is collected over water also in a mixture, the partial pressure of gas. The three fundamental gas laws discover the relationship of pressure, temperature, volume. Chemistry Gas Laws Cheat Sheet.
From scienceducatio.weebly.com
Gas Laws Environmental Chemistry Chemistry Gas Laws Cheat Sheet After the rates of diffusion/effusion for two gases are determined,. When a gas is collected over water also in a mixture, the partial pressure of gas. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant. Chemistry Gas Laws Cheat Sheet.
From www.slideshare.net
Temperature and Theory of Gases cheat sheet Chemistry Gas Laws Cheat Sheet When a gas is collected over water also in a mixture, the partial pressure of gas. The most energetic of the three states of matter, gases, has several laws that describe behavior. Calculate the total pressure in the. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new. Chemistry Gas Laws Cheat Sheet.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Chemistry Gas Laws Cheat Sheet 1 = mole fraction of gas. The most energetic of the three states of matter, gases, has several laws that describe behavior. The gas with the lower molar mass will be the. When a gas is collected over water also in a mixture, the partial pressure of gas. The kinetic molecular theory attempts to explain gas behavior. The ideal gas. Chemistry Gas Laws Cheat Sheet.
From www.chegg.com
Solved Ideal gas Gas equation pV = nRT = NkgT. Speed of Chemistry Gas Laws Cheat Sheet The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The gas with the lower molar mass will be the. The kinetic molecular theory attempts to explain gas behavior. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas law reduces. Calculate. Chemistry Gas Laws Cheat Sheet.
From www.chegg.com
Solved CHEMISTRY GAS LAW'S WORKSHEET Boyle's Law Charles' Chemistry Gas Laws Cheat Sheet The most energetic of the three states of matter, gases, has several laws that describe behavior. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. After the rates of diffusion/effusion for two gases are determined,. If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml,. Chemistry Gas Laws Cheat Sheet.
From www.scribd.com
Gas Laws Cheat Sheet PDF Chemistry Gas Laws Cheat Sheet If a gas has a pressure of 2.35 atm at 25oc, and fills a container of 543 ml, what is the new pressure if the container is. 1 = mole fraction of gas. The most energetic of the three states of matter, gases, has several laws that describe behavior. The gas with the lower molar mass will be the. After. Chemistry Gas Laws Cheat Sheet.
From pdfslide.net
Gas Law Cheat Sheet Chemistry Gas Laws Cheat Sheet The gas with the lower molar mass will be the. When a gas is collected over water also in a mixture, the partial pressure of gas. The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas law reduces. Calculate the total pressure in the. 1 = mole fraction. Chemistry Gas Laws Cheat Sheet.
From mathformula5.netlify.app
What Is The Mathematical Formula Of Boyles Law Complete Guide Chemistry Gas Laws Cheat Sheet The ideal gas law relates the pressure, temperature, volume and moles of a gas through the gas constant “r.” the ideal gas law reduces. The gas with the lower molar mass will be the. The most energetic of the three states of matter, gases, has several laws that describe behavior. After the rates of diffusion/effusion for two gases are determined,.. Chemistry Gas Laws Cheat Sheet.