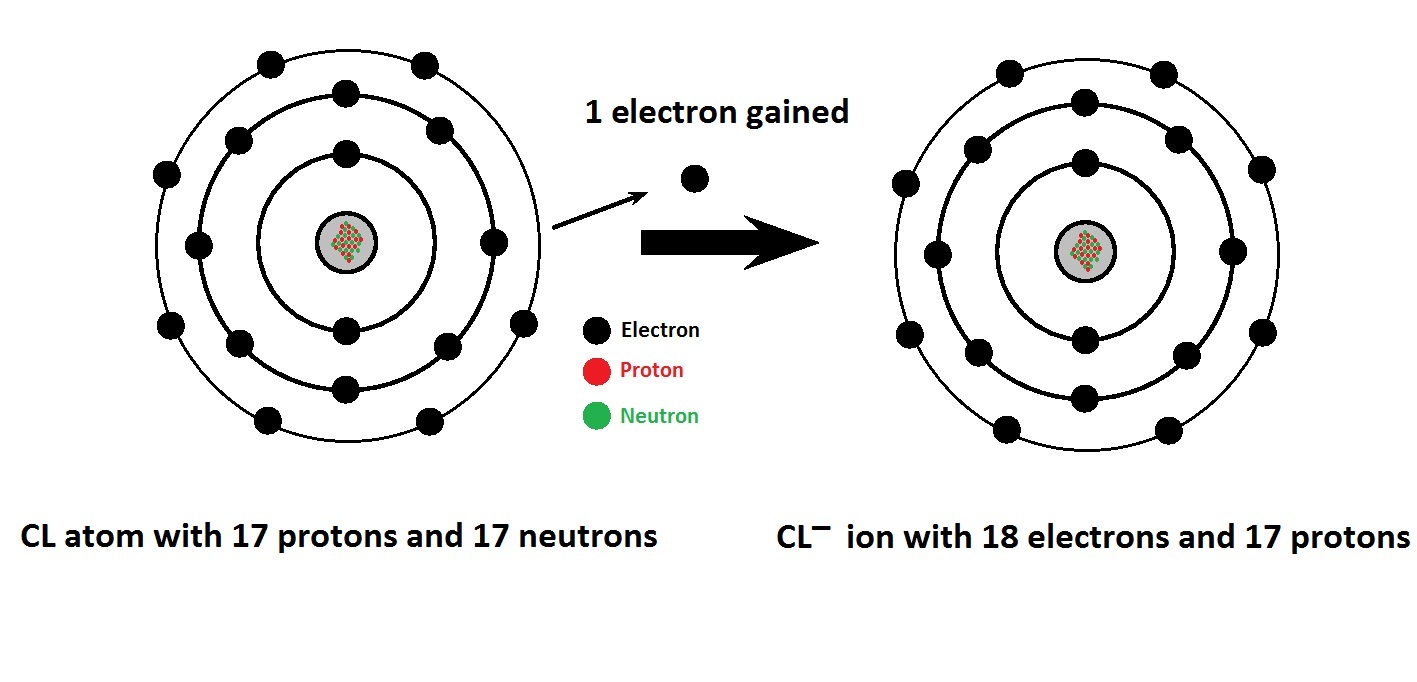
Chlorine And Lithium Chloride Reaction . This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. Chlorine + potassium iodide → potassium. It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. For example, chlorine is more reactive than iodine. Chlorine is more reactive than the iodine in potassium iodide solution: Halogens can be dissolved in water to form acidic solutions. The colourless solution changes to brown. For each element, we check if the number of atoms is balanced on both sides of the. A solution of chlorine can displace iodine from potassium iodide solution: Let's balance this equation using the inspection method. A more reactive halogen can displace a less reactive one from a compound.
from basichemistry.blogspot.com
The colourless solution changes to brown. Halogens can be dissolved in water to form acidic solutions. A solution of chlorine can displace iodine from potassium iodide solution: For example, chlorine is more reactive than iodine. Chlorine + potassium iodide → potassium. It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. Chlorine is more reactive than the iodine in potassium iodide solution: This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. Let's balance this equation using the inspection method. A more reactive halogen can displace a less reactive one from a compound.
Basic Chemistry October 2012
Chlorine And Lithium Chloride Reaction Halogens can be dissolved in water to form acidic solutions. Chlorine + potassium iodide → potassium. Let's balance this equation using the inspection method. It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. Halogens can be dissolved in water to form acidic solutions. A solution of chlorine can displace iodine from potassium iodide solution: The colourless solution changes to brown. For each element, we check if the number of atoms is balanced on both sides of the. Chlorine is more reactive than the iodine in potassium iodide solution: This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. A more reactive halogen can displace a less reactive one from a compound. For example, chlorine is more reactive than iodine.
From www.researchgate.net
Scheme of the spatial progress of lithium chloride intercalation into... Download Scientific Chlorine And Lithium Chloride Reaction This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. For example, chlorine is more reactive than iodine. For each element, we check if the number of atoms is balanced on both sides of the. A solution of chlorine can displace iodine from potassium iodide solution: Chlorine is more reactive than the. Chlorine And Lithium Chloride Reaction.
From slideplayer.com
Alkali Metals Electrostructure and reactivity Physical properties ppt download Chlorine And Lithium Chloride Reaction Chlorine + potassium iodide → potassium. Halogens can be dissolved in water to form acidic solutions. Let's balance this equation using the inspection method. The colourless solution changes to brown. For example, chlorine is more reactive than iodine. A solution of chlorine can displace iodine from potassium iodide solution: Chlorine is more reactive than the iodine in potassium iodide solution:. Chlorine And Lithium Chloride Reaction.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Molecules Combine with Chlorine And Lithium Chloride Reaction This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. A more reactive halogen can displace a less reactive one from a compound. Chlorine + potassium iodide → potassium. A solution of chlorine can displace iodine from potassium iodide solution: It is the highly exothermic reaction of lithium metal with either chlorine. Chlorine And Lithium Chloride Reaction.
From www.istockphoto.com
Sodium Chloride Formation Illustration Reaction Of Sodium With Chlorine Stock Illustration Chlorine And Lithium Chloride Reaction This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. Chlorine is more reactive than the iodine in potassium iodide solution: For each element, we check if the number of atoms is balanced on both sides of the. Halogens can be dissolved in water to form acidic solutions. The colourless solution changes. Chlorine And Lithium Chloride Reaction.
From www.numerade.com
SOLVED An atom of lithium (Li) forms an ionic bond with an atom of chlorine (Cl) to form Chlorine And Lithium Chloride Reaction Chlorine is more reactive than the iodine in potassium iodide solution: Let's balance this equation using the inspection method. A more reactive halogen can displace a less reactive one from a compound. The colourless solution changes to brown. Chlorine + potassium iodide → potassium. For each element, we check if the number of atoms is balanced on both sides of. Chlorine And Lithium Chloride Reaction.
From www.animalia-life.club
Lithium Chloride Bonding Chlorine And Lithium Chloride Reaction Chlorine is more reactive than the iodine in potassium iodide solution: It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. A more reactive halogen can displace a less reactive one from a compound. Halogens can be dissolved in water to form acidic solutions. For example, chlorine is more reactive than iodine. Chlorine. Chlorine And Lithium Chloride Reaction.
From m20131000606.blogspot.com
Chemistry Reactivity of Alkali Metal with Chlorine, Oxygen and Water Chlorine And Lithium Chloride Reaction Halogens can be dissolved in water to form acidic solutions. Let's balance this equation using the inspection method. This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. For each element, we check if the number of atoms is balanced on both sides of the. The colourless solution changes to brown. A. Chlorine And Lithium Chloride Reaction.
From courses.lumenlearning.com
4.2 Classifying Chemical Reactions Chemistry Chlorine And Lithium Chloride Reaction Chlorine is more reactive than the iodine in potassium iodide solution: It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. A more reactive halogen can displace a less reactive one from a compound. Chlorine + potassium iodide → potassium. Halogens can be dissolved in water to form acidic solutions. The colourless solution. Chlorine And Lithium Chloride Reaction.
From www.researchgate.net
10. Specific heat of lithium chloridewater Download Scientific Diagram Chlorine And Lithium Chloride Reaction Chlorine + potassium iodide → potassium. A solution of chlorine can displace iodine from potassium iodide solution: Let's balance this equation using the inspection method. For each element, we check if the number of atoms is balanced on both sides of the. The colourless solution changes to brown. This page examines the reactions of the group 1 elements (lithium, sodium,. Chlorine And Lithium Chloride Reaction.
From root469.blogspot.com
Ionic Bonding IGCSE Chemistry (0620) Best Notes Chlorine And Lithium Chloride Reaction It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. Chlorine + potassium iodide → potassium. Halogens can be dissolved in water to form acidic solutions. The colourless solution changes to brown. A solution of chlorine can displace iodine from potassium iodide solution: This page examines the reactions of the group 1 elements. Chlorine And Lithium Chloride Reaction.
From www.alamy.com
Synthesis reaction sodium chloride formation of sodium metal and chlorine gas. Types of Chlorine And Lithium Chloride Reaction Chlorine is more reactive than the iodine in potassium iodide solution: Let's balance this equation using the inspection method. Chlorine + potassium iodide → potassium. For each element, we check if the number of atoms is balanced on both sides of the. The colourless solution changes to brown. Halogens can be dissolved in water to form acidic solutions. For example,. Chlorine And Lithium Chloride Reaction.
From www.youtube.com
How to Balance Li + Cl2 = LiCl (Lithium + Chlorine gas) YouTube Chlorine And Lithium Chloride Reaction Chlorine + potassium iodide → potassium. Let's balance this equation using the inspection method. This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. Chlorine is more reactive than the iodine in potassium iodide solution: For example, chlorine is more reactive than iodine. For each element, we check if the number of. Chlorine And Lithium Chloride Reaction.
From www.coursehero.com
[Solved] Solid lithium chloride into solid lithium and chlorine... Course Hero Chlorine And Lithium Chloride Reaction A solution of chlorine can displace iodine from potassium iodide solution: Halogens can be dissolved in water to form acidic solutions. The colourless solution changes to brown. Let's balance this equation using the inspection method. This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. Chlorine is more reactive than the iodine. Chlorine And Lithium Chloride Reaction.
From www.numerade.com
SOLVED Solid lithium and chlorine gas are formed by the of solid lithium chloride Chlorine And Lithium Chloride Reaction For each element, we check if the number of atoms is balanced on both sides of the. Let's balance this equation using the inspection method. This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. Chlorine is more reactive than the iodine in potassium iodide solution: A solution of chlorine can displace. Chlorine And Lithium Chloride Reaction.
From www.animalia-life.club
Lithium Chloride Bonding Chlorine And Lithium Chloride Reaction Chlorine + potassium iodide → potassium. A more reactive halogen can displace a less reactive one from a compound. It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. The colourless solution changes to brown. Chlorine is more reactive than the iodine in potassium iodide solution: A solution of chlorine can displace iodine. Chlorine And Lithium Chloride Reaction.
From www.slideserve.com
PPT Ions and Ionic Bonds PowerPoint Presentation, free download ID3903745 Chlorine And Lithium Chloride Reaction The colourless solution changes to brown. This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. Halogens can be dissolved in water to form acidic solutions. Chlorine is more reactive than the iodine in potassium iodide solution: It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen. Chlorine And Lithium Chloride Reaction.
From brainly.in
This question is about metal compounds. (a) Lithium reacts with chlorine to produce lithium Chlorine And Lithium Chloride Reaction For example, chlorine is more reactive than iodine. For each element, we check if the number of atoms is balanced on both sides of the. Let's balance this equation using the inspection method. A solution of chlorine can displace iodine from potassium iodide solution: This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium). Chlorine And Lithium Chloride Reaction.
From www.pinterest.jp
Pin on Lífræn efnafræði Chlorine And Lithium Chloride Reaction Halogens can be dissolved in water to form acidic solutions. Let's balance this equation using the inspection method. This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. A more reactive halogen can displace a less reactive one from a compound. For each element, we check if the number of atoms is. Chlorine And Lithium Chloride Reaction.
From basichemistry.blogspot.com
Basic Chemistry October 2012 Chlorine And Lithium Chloride Reaction Chlorine + potassium iodide → potassium. A more reactive halogen can displace a less reactive one from a compound. A solution of chlorine can displace iodine from potassium iodide solution: It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. For example, chlorine is more reactive than iodine. Halogens can be dissolved in. Chlorine And Lithium Chloride Reaction.
From www.numerade.com
SOLVEDQ When lithiur reacts with chlorine the following reaction occurs 2Li + CL 2LiCI What is Chlorine And Lithium Chloride Reaction A more reactive halogen can displace a less reactive one from a compound. Chlorine is more reactive than the iodine in potassium iodide solution: A solution of chlorine can displace iodine from potassium iodide solution: This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. Chlorine + potassium iodide → potassium. The. Chlorine And Lithium Chloride Reaction.
From ar.inspiredpencil.com
Lithium Chloride Lewis Structure Chlorine And Lithium Chloride Reaction This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. For each element, we check if the number of atoms is balanced on both sides of the. It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. For example, chlorine is more reactive than iodine.. Chlorine And Lithium Chloride Reaction.
From pubs.acs.org
Chlorination of Aliphatic Primary Alcohols via Activation Organic Chlorine And Lithium Chloride Reaction Halogens can be dissolved in water to form acidic solutions. Chlorine is more reactive than the iodine in potassium iodide solution: It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. A solution of chlorine can displace iodine from potassium iodide solution: For example, chlorine is more reactive than iodine. A more reactive. Chlorine And Lithium Chloride Reaction.
From www.mindomo.com
Organic Reactions Mind Map Chlorine And Lithium Chloride Reaction The colourless solution changes to brown. This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. Chlorine is more reactive than the iodine in potassium iodide solution: A solution of chlorine can displace iodine from. Chlorine And Lithium Chloride Reaction.
From www.toppr.com
Lithium Chloride Formula Definition, Concepts and Examples Chlorine And Lithium Chloride Reaction A more reactive halogen can displace a less reactive one from a compound. Chlorine + potassium iodide → potassium. It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. For each element, we check if the number of atoms is balanced on both sides of the. Halogens can be dissolved in water to. Chlorine And Lithium Chloride Reaction.
From www.britannica.com
Chemical reaction The BrønstedLowry theory Britannica Chlorine And Lithium Chloride Reaction A solution of chlorine can displace iodine from potassium iodide solution: It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. Let's balance this equation using the inspection method. The colourless solution changes to brown. Chlorine is more reactive than the iodine in potassium iodide solution: Halogens can be dissolved in water to. Chlorine And Lithium Chloride Reaction.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Chlorine And Lithium Chloride Reaction Chlorine + potassium iodide → potassium. Let's balance this equation using the inspection method. For each element, we check if the number of atoms is balanced on both sides of the. The colourless solution changes to brown. Chlorine is more reactive than the iodine in potassium iodide solution: For example, chlorine is more reactive than iodine. This page examines the. Chlorine And Lithium Chloride Reaction.
From saylordotorg.github.io
Ions Chlorine And Lithium Chloride Reaction A more reactive halogen can displace a less reactive one from a compound. For each element, we check if the number of atoms is balanced on both sides of the. This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. Halogens can be dissolved in water to form acidic solutions. Let's balance. Chlorine And Lithium Chloride Reaction.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Chlorine And Lithium Chloride Reaction Chlorine is more reactive than the iodine in potassium iodide solution: Let's balance this equation using the inspection method. For example, chlorine is more reactive than iodine. For each element, we check if the number of atoms is balanced on both sides of the. This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium). Chlorine And Lithium Chloride Reaction.
From pediaa.com
Difference Between Chlorine and Chloride Definition, Properties, Reactions, Applications Chlorine And Lithium Chloride Reaction Chlorine is more reactive than the iodine in potassium iodide solution: For each element, we check if the number of atoms is balanced on both sides of the. The colourless solution changes to brown. This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. It is the highly exothermic reaction of lithium. Chlorine And Lithium Chloride Reaction.
From www.slideserve.com
PPT Chemistry Reaction Types PowerPoint Presentation, free download ID6906108 Chlorine And Lithium Chloride Reaction Chlorine is more reactive than the iodine in potassium iodide solution: The colourless solution changes to brown. Halogens can be dissolved in water to form acidic solutions. For example, chlorine is more reactive than iodine. A solution of chlorine can displace iodine from potassium iodide solution: A more reactive halogen can displace a less reactive one from a compound. Chlorine. Chlorine And Lithium Chloride Reaction.
From www.slideshare.net
Chemical Reactions Chlorine And Lithium Chloride Reaction A solution of chlorine can displace iodine from potassium iodide solution: For each element, we check if the number of atoms is balanced on both sides of the. A more reactive halogen can displace a less reactive one from a compound. This page examines the reactions of the group 1 elements (lithium, sodium, potassium, rubidium and cesium) with with. Halogens. Chlorine And Lithium Chloride Reaction.
From www.researchgate.net
The lithiumchlorine pair correlation functions of aqueous LiCl... Download Scientific Diagram Chlorine And Lithium Chloride Reaction Let's balance this equation using the inspection method. It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. Halogens can be dissolved in water to form acidic solutions. The colourless solution changes to brown. For example, chlorine is more reactive than iodine. For each element, we check if the number of atoms is. Chlorine And Lithium Chloride Reaction.
From www.vrogue.co
Lithium Chloride Dot And Cross Diagram This Is How Th vrogue.co Chlorine And Lithium Chloride Reaction The colourless solution changes to brown. Let's balance this equation using the inspection method. A more reactive halogen can displace a less reactive one from a compound. For example, chlorine is more reactive than iodine. Chlorine is more reactive than the iodine in potassium iodide solution: Halogens can be dissolved in water to form acidic solutions. For each element, we. Chlorine And Lithium Chloride Reaction.
From www.animalia-life.club
Lithium Chloride Bonding Chlorine And Lithium Chloride Reaction For each element, we check if the number of atoms is balanced on both sides of the. Chlorine is more reactive than the iodine in potassium iodide solution: For example, chlorine is more reactive than iodine. It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. A more reactive halogen can displace a. Chlorine And Lithium Chloride Reaction.
From www.youtube.com
Equation for LiCl + H2O (Lithium chloride + Water) YouTube Chlorine And Lithium Chloride Reaction Halogens can be dissolved in water to form acidic solutions. It is the highly exothermic reaction of lithium metal with either chlorine or anhydrous hydrogen chloride gas. For each element, we check if the number of atoms is balanced on both sides of the. A more reactive halogen can displace a less reactive one from a compound. A solution of. Chlorine And Lithium Chloride Reaction.