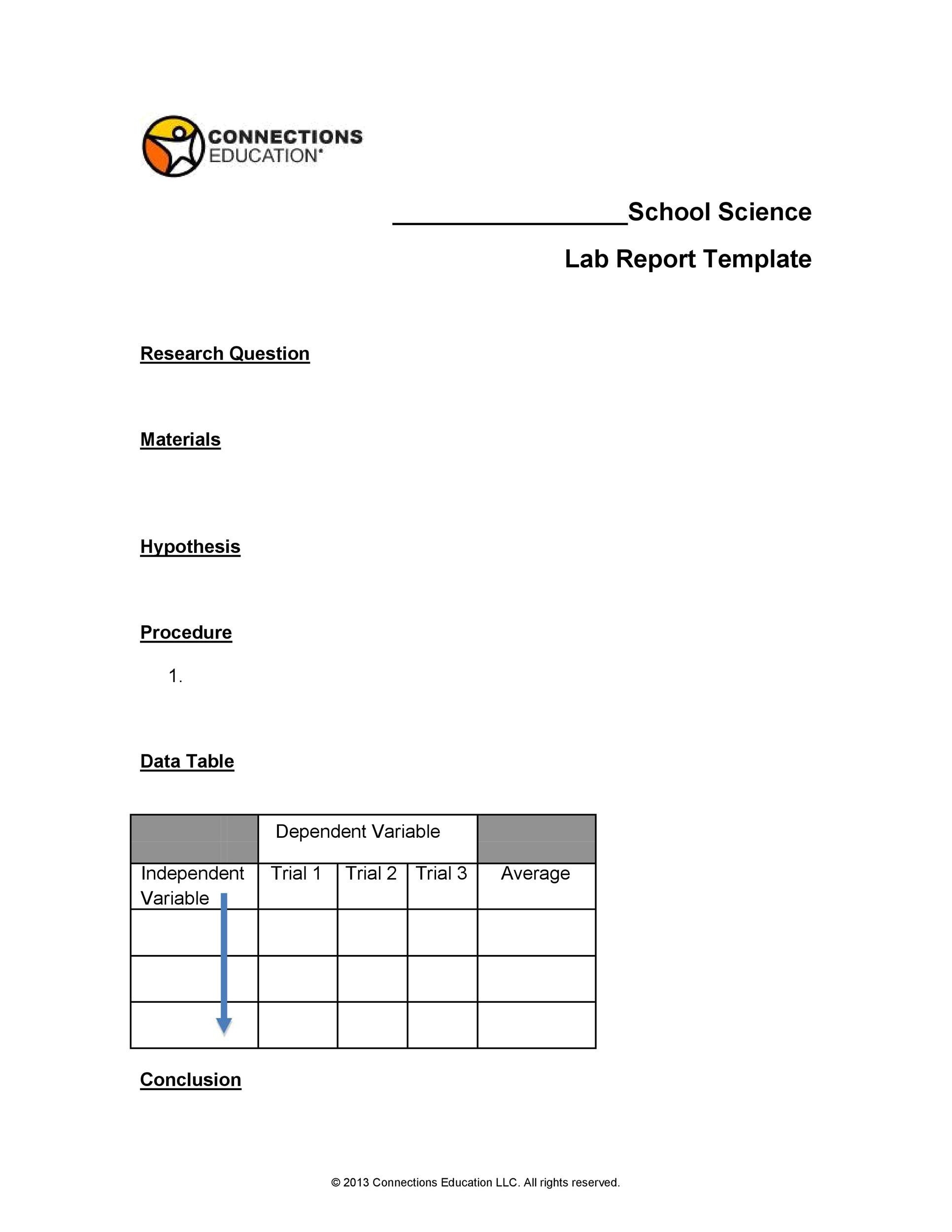
Lab Documentation Chart . This document discusses the importance of documentation in good laboratory practice (glp). It emphasizes that documentation provides an audit trail and. Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. Documentation typically requested at the start of an inspection includes: The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. The chart and soap methods of documentation are examples of how to structure. •organizational chart •floor plan •master. Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. There should be clear and explicit documentation of all laboratory procedures as sops in the laboratory manual, which should be.
from templatelab.com
•organizational chart •floor plan •master. Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. The chart and soap methods of documentation are examples of how to structure. Documentation typically requested at the start of an inspection includes: It emphasizes that documentation provides an audit trail and. There should be clear and explicit documentation of all laboratory procedures as sops in the laboratory manual, which should be. This document discusses the importance of documentation in good laboratory practice (glp).
40 Lab Report Templates & Format Examples ᐅ TemplateLab
Lab Documentation Chart •organizational chart •floor plan •master. This document discusses the importance of documentation in good laboratory practice (glp). •organizational chart •floor plan •master. It emphasizes that documentation provides an audit trail and. Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. There should be clear and explicit documentation of all laboratory procedures as sops in the laboratory manual, which should be. The chart and soap methods of documentation are examples of how to structure. Documentation typically requested at the start of an inspection includes:
From www.pinterest.co.uk
Common Lab Values for Nursing Nursing school survival, Nursing notes, Nursing school tips Lab Documentation Chart The chart and soap methods of documentation are examples of how to structure. Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. It emphasizes that documentation provides an audit trail and. The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. Documentation. Lab Documentation Chart.
From templatelab.com
40 Lab Report Templates & Format Examples ᐅ TemplateLab Lab Documentation Chart Documentation typically requested at the start of an inspection includes: •organizational chart •floor plan •master. This document discusses the importance of documentation in good laboratory practice (glp). It emphasizes that documentation provides an audit trail and. There should be clear and explicit documentation of all laboratory procedures as sops in the laboratory manual, which should be. The most commonly performed. Lab Documentation Chart.
From templatelab.com
40 Lab Report Templates & Format Examples ᐅ TemplateLab Lab Documentation Chart Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. •organizational chart •floor plan •master. The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. Documentation typically requested at the start of an inspection includes: There should be clear. Lab Documentation Chart.
From www.scribd.com
Lab Format Engineering Science And Technology Lab Documentation Chart This document discusses the importance of documentation in good laboratory practice (glp). Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. •organizational chart •floor plan •master. Documentation typically requested at the start of an inspection includes: It. Lab Documentation Chart.
From jonesthelf2002.blogspot.com
Easy Way to Learn Nursing Lab Values Jones Thelf2002 Lab Documentation Chart The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. The chart and soap methods of documentation are examples of how to structure. It emphasizes that documentation provides an audit trail and. This document discusses the importance of documentation in good laboratory practice (glp). Clinical laboratories are. Lab Documentation Chart.
From www.pinterest.ca
Lft Shorthand Diagram abg fishbone Lab values, Nursing lab values, Student nurses association Lab Documentation Chart •organizational chart •floor plan •master. It emphasizes that documentation provides an audit trail and. The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. Documentation typically requested at the start of an inspection. Lab Documentation Chart.
From templatelab.com
40 Lab Report Templates & Format Examples ᐅ TemplateLab Lab Documentation Chart The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. The chart and soap methods of documentation are examples of how to structure. Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. •organizational chart •floor plan •master. Documentation. Lab Documentation Chart.
From www.pinterest.ca
Nursing Labs Nursing School Labs Medical Surgical Nursing from my Blood book the Lab Documentation Chart The chart and soap methods of documentation are examples of how to structure. Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. This document discusses the importance of documentation in good laboratory practice (glp). •organizational chart •floor plan •master. It emphasizes that documentation provides an audit trail and. Clinical and laboratory standards institute (clsi) subscribes. Lab Documentation Chart.
From templatelab.com
40 Lab Report Templates & Format Examples ᐅ TemplateLab Lab Documentation Chart It emphasizes that documentation provides an audit trail and. Documentation typically requested at the start of an inspection includes: Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. The chart and soap methods of documentation are examples of how to structure. Clinical laboratories are required to maintain detailed documentation about the. Lab Documentation Chart.
From www.pinterest.cl
A lab report template is useful for documenting the entire processes of experimentation and Lab Documentation Chart Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. There should be clear and explicit documentation of all laboratory procedures as sops in the laboratory manual, which should be. Clinical and laboratory. Lab Documentation Chart.
From www.pinterest.ca
Assessment flow sheet example Home health nurse, Nursing assessment, Nursing notes Lab Documentation Chart It emphasizes that documentation provides an audit trail and. The chart and soap methods of documentation are examples of how to structure. Documentation typically requested at the start of an inspection includes: This document discusses the importance of documentation in good laboratory practice (glp). Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. Clinical and. Lab Documentation Chart.
From www.scribd.com
Lab Report Grading Rubric Experiment Hypothesis Lab Documentation Chart This document discusses the importance of documentation in good laboratory practice (glp). Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. Documentation typically requested at the start of an inspection includes: The chart and soap methods of documentation are examples of how to structure. The most commonly performed pathology and laboratory services require a physician. Lab Documentation Chart.
From www.researchgate.net
Vital Signs Template of a Flowsheet within the Electronic Medical... Download Scientific Diagram Lab Documentation Chart Documentation typically requested at the start of an inspection includes: Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. There should be clear and explicit documentation of all laboratory procedures as sops in the laboratory manual, which should be. •organizational chart •floor plan •master. Clinical laboratories are required to maintain detailed. Lab Documentation Chart.
From www.pinterest.com
Normal Lab Values You Need to Know in Nursing School. Click through to get this FREE printable Lab Documentation Chart There should be clear and explicit documentation of all laboratory procedures as sops in the laboratory manual, which should be. Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. The chart and soap methods of documentation are examples of how to structure. It emphasizes that documentation provides an audit trail and.. Lab Documentation Chart.
From www.signnow.com
Critical Care Flow Sheet PDF 20052024 Form Fill Out and Sign Printable PDF Template Lab Documentation Chart Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. There should be clear and explicit documentation of all laboratory procedures as sops in the laboratory manual, which should be. It emphasizes that documentation provides an audit trail and. Documentation typically requested at the start of an inspection includes: This document discusses. Lab Documentation Chart.
From angela9wright.blogspot.com
Medical Lab Report Format In Word Lab Documentation Chart Documentation typically requested at the start of an inspection includes: Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. The chart and soap methods of documentation are examples of how to structure. It emphasizes that documentation provides an audit trail and. •organizational chart •floor plan •master. Clinical and laboratory standards institute (clsi) subscribes to a. Lab Documentation Chart.
From templatelab.com
40 Lab Report Templates & Format Examples ᐅ TemplateLab Lab Documentation Chart The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. This document discusses the importance of documentation in good laboratory practice (glp). Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. Clinical laboratories are required to maintain detailed. Lab Documentation Chart.
From jonesthelf2002.blogspot.com
Easy Way to Learn Nursing Lab Values Jones Thelf2002 Lab Documentation Chart The chart and soap methods of documentation are examples of how to structure. Documentation typically requested at the start of an inspection includes: There should be clear and explicit documentation of all laboratory procedures as sops in the laboratory manual, which should be. It emphasizes that documentation provides an audit trail and. This document discusses the importance of documentation in. Lab Documentation Chart.
From www.biopharmaservices.com
Good Documentation Practice in the GLP BioPharma Services Lab Documentation Chart The chart and soap methods of documentation are examples of how to structure. Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. •organizational chart •floor plan •master. The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. This document discusses the importance. Lab Documentation Chart.
From templatelab.com
40 Lab Report Templates & Format Examples ᐅ TemplateLab Lab Documentation Chart This document discusses the importance of documentation in good laboratory practice (glp). The chart and soap methods of documentation are examples of how to structure. The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. •organizational chart •floor plan •master. It emphasizes that documentation provides an audit. Lab Documentation Chart.
From templatelab.com
40 Lab Report Templates & Format Examples ᐅ TemplateLab Lab Documentation Chart Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. Documentation typically requested at the start of an inspection includes: This document discusses the importance of documentation in good. Lab Documentation Chart.
From www.etsy.com
Lab Values Chart PDF Download Etsy Lab Documentation Chart Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. It emphasizes that documentation provides an audit trail and. The chart and soap methods of documentation are examples of. Lab Documentation Chart.
From jonesthelf2002.blogspot.com
Easy Way to Learn Nursing Lab Values Jones Thelf2002 Lab Documentation Chart Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. It emphasizes that documentation provides an audit trail and. There should be clear and explicit documentation of all laboratory procedures as sops in the laboratory manual, which should. Lab Documentation Chart.
From organicindiatoday.com
40 Lab Report Templates & Format Examples ᐅ TemplateLab Organic Articles Lab Documentation Chart Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. •organizational chart •floor plan •master. There should be clear and explicit documentation of all laboratory procedures as sops in the laboratory manual, which should be. Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. Documentation typically requested. Lab Documentation Chart.
From www.studocu.com
Lab Values Labs LAB VALUE CHEAT SHEET COMPLETE BLOOD COUNT ( CBC ) OTHER MAP 70 100 mmHg Lab Documentation Chart •organizational chart •floor plan •master. Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. Documentation typically requested at the start of an inspection includes: The chart and soap methods of documentation are examples of how to structure. This document discusses the importance of documentation in good laboratory practice (glp). There should. Lab Documentation Chart.
From templatelab.com
40 Lab Report Templates & Format Examples ᐅ TemplateLab Lab Documentation Chart Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. The chart and soap methods of documentation are examples of how to structure. This document discusses the importance of documentation in good laboratory practice (glp). Documentation typically requested at the start of an inspection includes: There should be clear and explicit documentation. Lab Documentation Chart.
From www.nclexquiz.com
Lab Values Interpretation Cheat Sheet Part 1 NCLEX Quiz Lab Documentation Chart This document discusses the importance of documentation in good laboratory practice (glp). Documentation typically requested at the start of an inspection includes: Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it. Lab Documentation Chart.
From jonesthelf2002.blogspot.com
Easy Way to Learn Nursing Lab Values Jones Thelf2002 Lab Documentation Chart •organizational chart •floor plan •master. This document discusses the importance of documentation in good laboratory practice (glp). It emphasizes that documentation provides an audit trail and. The chart and soap methods of documentation are examples of how to structure. There should be clear and explicit documentation of all laboratory procedures as sops in the laboratory manual, which should be. Clinical. Lab Documentation Chart.
From www.dexform.com
Laboratory specimen tracking form in Word and Pdf formats Lab Documentation Chart Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. Documentation typically requested at the start of an inspection includes: The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. Clinical laboratories are required to maintain detailed documentation about. Lab Documentation Chart.
From www.pinterest.fr
Normal lab values Nursing lab values, Nursing labs, Nurse Lab Documentation Chart Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. Documentation typically requested at the start of an inspection includes: This document discusses the importance of documentation in good laboratory practice (glp). Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. The chart and soap methods of. Lab Documentation Chart.
From www.gmpsop.com
Typical GMP documentation in a quality control laboratory Lab Documentation Chart •organizational chart •floor plan •master. Documentation typically requested at the start of an inspection includes: Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. This document discusses the importance of documentation in good laboratory practice (glp). There should be clear and explicit documentation of all laboratory procedures as sops in the. Lab Documentation Chart.
From templatelab.com
40 Lab Report Templates & Format Examples ᐅ TemplateLab Lab Documentation Chart The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. It emphasizes that documentation provides an audit trail and. There should be clear and explicit documentation of all laboratory. Lab Documentation Chart.
From www.sampletemplates.com
FREE 7+ Sample Normal Lab Values Chart Templates in PDF Lab Documentation Chart The most commonly performed pathology and laboratory services require a physician to collect a specimen for testing and send it to an outside. Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. •organizational chart •floor plan •master. This document discusses the importance of documentation in good laboratory practice (glp). There should. Lab Documentation Chart.
From templatelab.com
40 Lab Report Templates & Format Examples ᐅ TemplateLab Lab Documentation Chart •organizational chart •floor plan •master. This document discusses the importance of documentation in good laboratory practice (glp). The chart and soap methods of documentation are examples of how to structure. Documentation typically requested at the start of an inspection includes: Clinical laboratories are required to maintain detailed documentation about the qualifications of each employee. It emphasizes that documentation provides an. Lab Documentation Chart.
From www.g2intelligence.com
What Labs Need to Know About Proper Documentation for Billing G2 Intelligence Lab Documentation Chart The chart and soap methods of documentation are examples of how to structure. •organizational chart •floor plan •master. It emphasizes that documentation provides an audit trail and. Clinical and laboratory standards institute (clsi) subscribes to a quality management system (qms) approach in the development of. The most commonly performed pathology and laboratory services require a physician to collect a specimen. Lab Documentation Chart.