Iron(Ii) Oxide In Water . Iron oxides feature as ferrous (fe (ii)) or ferric (fe (iii)) or both. Technically, it's iron oxide hydrate, because pure iron oxide isn't rust. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Iron (ii) oxide is not soluble in water, but it is in strong acids. Iron forms three different oxides: The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. Wustite is a naturally occurring mineral with iron (ii) oxide in it. They adopt octahedral or tetrahedral coordination geometry. Iron(ii) oxide can be formed from the. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. The zeta potential of iron oxide. Rusting occurs when iron or its alloys are exposed. Only a few oxides are significant at the earth's surface, particularly. Rust is the common name of the chemical called iron oxide.
from www.linstitute.net
Iron(ii) oxide can be formed from the. Iron forms three different oxides: The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Rust is the common name of the chemical called iron oxide. Rusting occurs when iron or its alloys are exposed. Technically, it's iron oxide hydrate, because pure iron oxide isn't rust. Iron (ii) oxide is not soluble in water, but it is in strong acids. They adopt octahedral or tetrahedral coordination geometry.
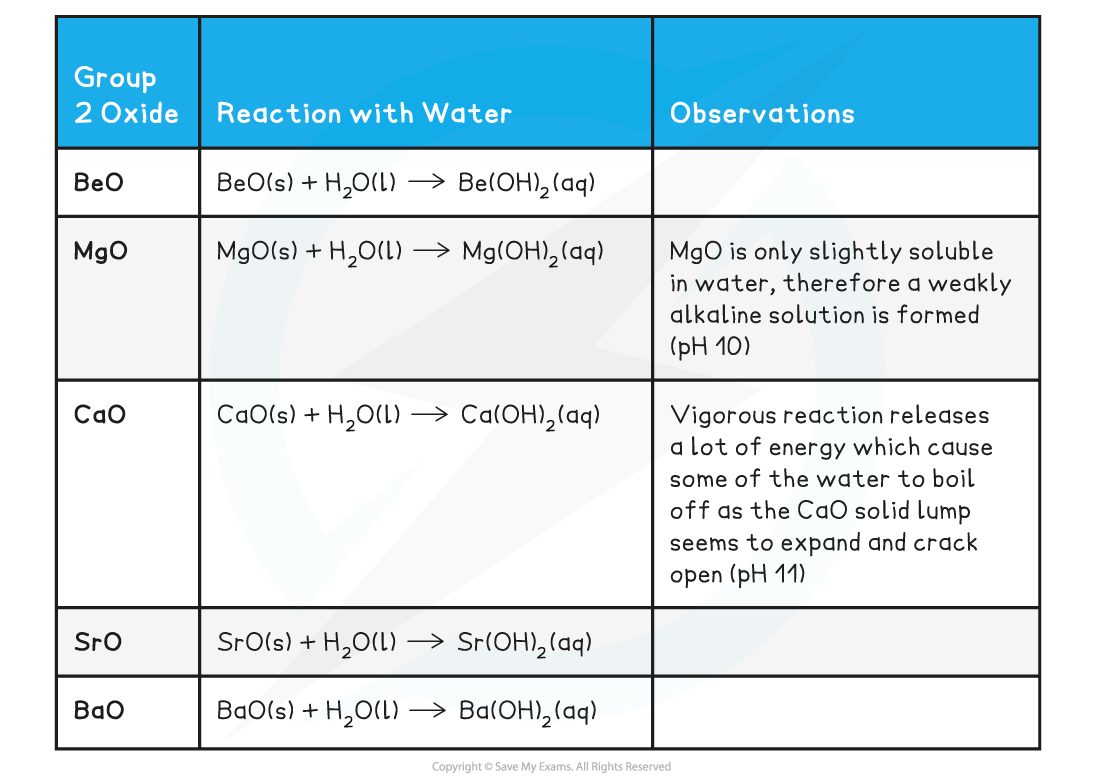
CIE A Level Chemistry复习笔记2.2.2 Reactions of Group 2 Oxides, Hydroxides
Iron(Ii) Oxide In Water Only a few oxides are significant at the earth's surface, particularly. The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. Rust is the common name of the chemical called iron oxide. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron forms three different oxides: Iron(ii) oxide can be formed from the. Iron (ii) oxide is not soluble in water, but it is in strong acids. Rusting occurs when iron or its alloys are exposed. Iron oxides feature as ferrous (fe (ii)) or ferric (fe (iii)) or both. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. They adopt octahedral or tetrahedral coordination geometry. The zeta potential of iron oxide. Technically, it's iron oxide hydrate, because pure iron oxide isn't rust. Wustite is a naturally occurring mineral with iron (ii) oxide in it. Only a few oxides are significant at the earth's surface, particularly.
From pubs.acs.org
Photoreductive Dissolution of Iron (Hydr)oxides and Its Geochemical Iron(Ii) Oxide In Water They adopt octahedral or tetrahedral coordination geometry. Rusting occurs when iron or its alloys are exposed. The zeta potential of iron oxide. The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from. Iron(Ii) Oxide In Water.
From askfilo.com
Metals react with water and produce a metal oxide and hydrogen gas. Metal.. Iron(Ii) Oxide In Water Rust is the common name of the chemical called iron oxide. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Iron(ii) oxide can be formed from the. The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. They adopt. Iron(Ii) Oxide In Water.
From www.ceramic-glazes.com
IRON OXIDE Iron (III) Oxide Minium Pigments and Stains Iron(Ii) Oxide In Water Iron forms three different oxides: They adopt octahedral or tetrahedral coordination geometry. Iron oxides feature as ferrous (fe (ii)) or ferric (fe (iii)) or both. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. The zeta potential of iron oxide. Rust is the common name of the chemical called iron oxide. The ph value of iron oxide in water typically ranges. Iron(Ii) Oxide In Water.
From www.carolina.com
Iron (II, III) Oxide, Black, Powder, Laboratory Grade, 500 g Carolina Iron(Ii) Oxide In Water Iron forms three different oxides: They adopt octahedral or tetrahedral coordination geometry. Iron(ii) oxide can be formed from the. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Rusting occurs when iron or its alloys are exposed. Wustite is a naturally. Iron(Ii) Oxide In Water.
From www.hanlin.com
CIE A Level Chemistry复习笔记2.1.3 Period 3 Oxides翰林国际教育 Iron(Ii) Oxide In Water Iron (ii) oxide is not soluble in water, but it is in strong acids. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron(ii) oxide can be formed from the. The ph value of iron oxide in water typically ranges from. Iron(Ii) Oxide In Water.
From www.linstitute.net
CIE A Level Chemistry复习笔记2.2.2 Reactions of Group 2 Oxides, Hydroxides Iron(Ii) Oxide In Water Rusting occurs when iron or its alloys are exposed. The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. Technically, it's iron oxide hydrate, because pure iron oxide isn't rust. Rust is the common name of the chemical called iron oxide. These microbes biologically oxidize the ferrous (fe 2+) iron. Iron(Ii) Oxide In Water.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Iron(Ii) Oxide In Water Iron(ii) oxide can be formed from the. Wustite is a naturally occurring mineral with iron (ii) oxide in it. Rust is the common name of the chemical called iron oxide. Technically, it's iron oxide hydrate, because pure iron oxide isn't rust. Iron oxides feature as ferrous (fe (ii)) or ferric (fe (iii)) or both. These microbes biologically oxidize the ferrous. Iron(Ii) Oxide In Water.
From www.researchgate.net
Mössbauer parameters of iron oxides and hydroxides. Download Table Iron(Ii) Oxide In Water The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Rusting occurs when iron or its alloys are exposed. The zeta potential of iron oxide. Rust is the common name. Iron(Ii) Oxide In Water.
From www.youtube.com
How to write the equation for FeO + H2O Iron (II) oxide + Water YouTube Iron(Ii) Oxide In Water The zeta potential of iron oxide. Wustite is a naturally occurring mineral with iron (ii) oxide in it. Only a few oxides are significant at the earth's surface, particularly. The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. Iron forms three different oxides: Iron(ii) oxide can be formed from. Iron(Ii) Oxide In Water.
From www.amertek.co.uk
Iron (II) Oxide (Black) / Ferrous Oxide FeO Good Grade Powder (Iron Iron(Ii) Oxide In Water These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Iron(ii) oxide can be formed from the. The zeta potential of iron oxide. Only a few oxides are significant at the earth's surface, particularly. Wustite is a naturally occurring mineral with iron (ii) oxide in it. Rust is the common. Iron(Ii) Oxide In Water.
From www.alamy.com
Rust is an iron oxide, a usually reddishbrown oxide formed by the Iron(Ii) Oxide In Water Wustite is a naturally occurring mineral with iron (ii) oxide in it. Rust is the common name of the chemical called iron oxide. Iron(ii) oxide can be formed from the. They adopt octahedral or tetrahedral coordination geometry. Rusting occurs when iron or its alloys are exposed. Iron oxides feature as ferrous (fe (ii)) or ferric (fe (iii)) or both. Only. Iron(Ii) Oxide In Water.
From www.reddit.com
Iron (III) Oxide precipitate from my lab today. I thought it looked Iron(Ii) Oxide In Water Iron(ii) oxide can be formed from the. Iron oxides feature as ferrous (fe (ii)) or ferric (fe (iii)) or both. Only a few oxides are significant at the earth's surface, particularly. Wustite is a naturally occurring mineral with iron (ii) oxide in it. Iron forms three different oxides: Rusting occurs when iron or its alloys are exposed. Iron(ii) oxide, iron(iii). Iron(Ii) Oxide In Water.
From www.vrogue.co
What Are The Respective Formulas For Iron Ii Oxide An vrogue.co Iron(Ii) Oxide In Water Wustite is a naturally occurring mineral with iron (ii) oxide in it. They adopt octahedral or tetrahedral coordination geometry. Technically, it's iron oxide hydrate, because pure iron oxide isn't rust. Iron (ii) oxide is not soluble in water, but it is in strong acids. Iron forms three different oxides: Iron oxides feature as ferrous (fe (ii)) or ferric (fe (iii)). Iron(Ii) Oxide In Water.
From www.tessshebaylo.com
Write A Word Equation To Represent The Process Of Rusting Iron Iron(Ii) Oxide In Water Only a few oxides are significant at the earth's surface, particularly. The zeta potential of iron oxide. The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. They adopt octahedral or tetrahedral coordination geometry. Iron(ii) oxide can be formed from the. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. These. Iron(Ii) Oxide In Water.
From www.sciencephoto.com
Iron (III) oxide in water and in hydrochloric acid Stock Image C036 Iron(Ii) Oxide In Water These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Iron forms three different oxides: Only a few oxides are significant at the earth's surface, particularly. Iron (ii) oxide is not soluble in water, but it is in strong acids. They adopt octahedral or tetrahedral coordination geometry. Iron(ii) oxide, iron(iii). Iron(Ii) Oxide In Water.
From noahchemicals.com
3 Manufacturing Applications for Iron (III) Oxide Noah Chemicals Iron(Ii) Oxide In Water The zeta potential of iron oxide. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Wustite is a naturally occurring mineral with iron (ii) oxide in it. Rusting occurs when iron or its alloys are exposed. Iron (ii) oxide is not soluble in water, but it is in strong. Iron(Ii) Oxide In Water.
From www.nagwa.com
Question Video Identifying Iron Oxide Produced from the Reaction of Iron(Ii) Oxide In Water Rusting occurs when iron or its alloys are exposed. The zeta potential of iron oxide. Iron forms three different oxides: They adopt octahedral or tetrahedral coordination geometry. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Technically, it's iron oxide hydrate, because pure iron oxide isn't rust. Iron (ii) oxide is not soluble in water, but it is in strong acids.. Iron(Ii) Oxide In Water.
From www.youtube.com
Gas stoichiometry of iron(III) hydroxide YouTube Iron(Ii) Oxide In Water Iron oxides feature as ferrous (fe (ii)) or ferric (fe (iii)) or both. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. Iron(ii) oxide can be formed from the. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe. Iron(Ii) Oxide In Water.
From www.hmicronpowder.com
Iron Oxide Hosokawa Micron Powder Systems Iron(Ii) Oxide In Water Iron forms three different oxides: Iron(ii) oxide can be formed from the. Technically, it's iron oxide hydrate, because pure iron oxide isn't rust. Only a few oxides are significant at the earth's surface, particularly. Iron oxides feature as ferrous (fe (ii)) or ferric (fe (iii)) or both. Iron (ii) oxide is not soluble in water, but it is in strong. Iron(Ii) Oxide In Water.
From lab.honeywell.com
Iron(III) oxide 12344 Honeywell Research Chemicals Iron(Ii) Oxide In Water Wustite is a naturally occurring mineral with iron (ii) oxide in it. The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. Iron (ii) oxide is not soluble in water, but it is in strong acids. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron forms three different oxides: Technically,. Iron(Ii) Oxide In Water.
From w20.b2m.cz
Fe2 Co3 3 H2so4 Fe2 So4 3 H2o Co2 EDUCA Iron(Ii) Oxide In Water Wustite is a naturally occurring mineral with iron (ii) oxide in it. They adopt octahedral or tetrahedral coordination geometry. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+). Iron(Ii) Oxide In Water.
From www.youtube.com
Iron(II) oxide YouTube Iron(Ii) Oxide In Water Only a few oxides are significant at the earth's surface, particularly. Iron(ii) oxide can be formed from the. The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Iron (ii). Iron(Ii) Oxide In Water.
From www.youtube.com
Expanding the Boundaries of Iron Oxide Color Spaces YouTube Iron(Ii) Oxide In Water Rusting occurs when iron or its alloys are exposed. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Wustite is a naturally occurring mineral with iron (ii) oxide in it. They adopt octahedral or tetrahedral coordination geometry. Iron forms three different oxides: Iron oxides feature as ferrous (fe (ii)). Iron(Ii) Oxide In Water.
From www.youtube.com
How to write the formula for iron (II) oxide YouTube Iron(Ii) Oxide In Water The zeta potential of iron oxide. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Iron forms three different oxides: Only a few oxides are significant at the earth's surface, particularly. Rust is the common name of the chemical called iron oxide. Iron(ii) oxide can be formed from the.. Iron(Ii) Oxide In Water.
From www.researchgate.net
UV/Visible spectra of iron oxide nanoparticle in water. Download Iron(Ii) Oxide In Water Rusting occurs when iron or its alloys are exposed. Iron (ii) oxide is not soluble in water, but it is in strong acids. They adopt octahedral or tetrahedral coordination geometry. Iron oxides feature as ferrous (fe (ii)) or ferric (fe (iii)) or both. Wustite is a naturally occurring mineral with iron (ii) oxide in it. Rust is the common name. Iron(Ii) Oxide In Water.
From testbook.com
Iron (II) Oxide Formula Structure, Preparation & Properties Iron(Ii) Oxide In Water The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Rust is the common name of the chemical called iron oxide. The zeta potential of iron oxide. Iron (ii) oxide. Iron(Ii) Oxide In Water.
From en.wikipedia.org
Iron(II) oxide Wikipedia Iron(Ii) Oxide In Water Rust is the common name of the chemical called iron oxide. Iron forms three different oxides: Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron (ii) oxide is not soluble in water, but it is in strong acids. Technically, it's iron oxide hydrate, because pure iron oxide isn't rust. Iron(ii) oxide can be formed from the. The ph value of. Iron(Ii) Oxide In Water.
From chemicaldb.netlify.app
Why does copper oxide not dissolve in water Iron(Ii) Oxide In Water Rusting occurs when iron or its alloys are exposed. Iron oxides feature as ferrous (fe (ii)) or ferric (fe (iii)) or both. Iron (ii) oxide is not soluble in water, but it is in strong acids. They adopt octahedral or tetrahedral coordination geometry. The zeta potential of iron oxide. Rust is the common name of the chemical called iron oxide.. Iron(Ii) Oxide In Water.
From www.thoughtco.com
Easy Steps for Balancing Chemical Equations Iron(Ii) Oxide In Water Iron (ii) oxide is not soluble in water, but it is in strong acids. Iron forms three different oxides: Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron(ii) oxide can be formed from the. Rusting occurs when iron or its alloys are exposed. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it. Iron(Ii) Oxide In Water.
From documentride5.pythonanywhere.com
How To Write Oxide Documentride5 Iron(Ii) Oxide In Water Iron(ii) oxide can be formed from the. Iron oxides feature as ferrous (fe (ii)) or ferric (fe (iii)) or both. They adopt octahedral or tetrahedral coordination geometry. Iron (ii) oxide is not soluble in water, but it is in strong acids. Rust is the common name of the chemical called iron oxide. Iron forms three different oxides: Only a few. Iron(Ii) Oxide In Water.
From www.youtube.com
How to Balance FeCl3 + Na2S = Fe2S3 + NaCl (Iron (III) chloride Iron(Ii) Oxide In Water Rusting occurs when iron or its alloys are exposed. Wustite is a naturally occurring mineral with iron (ii) oxide in it. These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. Iron (ii) oxide is not soluble in water, but it is in strong acids. Technically, it's iron oxide hydrate,. Iron(Ii) Oxide In Water.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) YouTube Iron(Ii) Oxide In Water Iron forms three different oxides: These microbes biologically oxidize the ferrous (fe 2+) iron to ferric (fe 3+) iron and remove it from the water. The zeta potential of iron oxide. Wustite is a naturally occurring mineral with iron (ii) oxide in it. Rust is the common name of the chemical called iron oxide. Rusting occurs when iron or its. Iron(Ii) Oxide In Water.
From www.youtube.com
Making Iron (II) Sulfide YouTube Iron(Ii) Oxide In Water Rusting occurs when iron or its alloys are exposed. Iron(ii) oxide can be formed from the. Technically, it's iron oxide hydrate, because pure iron oxide isn't rust. Wustite is a naturally occurring mineral with iron (ii) oxide in it. Iron forms three different oxides: The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an. Iron(Ii) Oxide In Water.
From priscillanewslara.blogspot.com
Molar Mass of Iron Ii Oxide Iron(Ii) Oxide In Water Technically, it's iron oxide hydrate, because pure iron oxide isn't rust. Iron forms three different oxides: Iron(ii) oxide can be formed from the. Iron (ii) oxide is not soluble in water, but it is in strong acids. The zeta potential of iron oxide. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Rusting occurs when iron or its alloys are exposed.. Iron(Ii) Oxide In Water.
From www.pw.live
Iron II Oxide Formula Structure, Properties, Uses Iron(Ii) Oxide In Water Iron (ii) oxide is not soluble in water, but it is in strong acids. The ph value of iron oxide in water typically ranges from 5.5 to 8.2, with an optimum level of 6.5. Only a few oxides are significant at the earth's surface, particularly. Technically, it's iron oxide hydrate, because pure iron oxide isn't rust. Rusting occurs when iron. Iron(Ii) Oxide In Water.