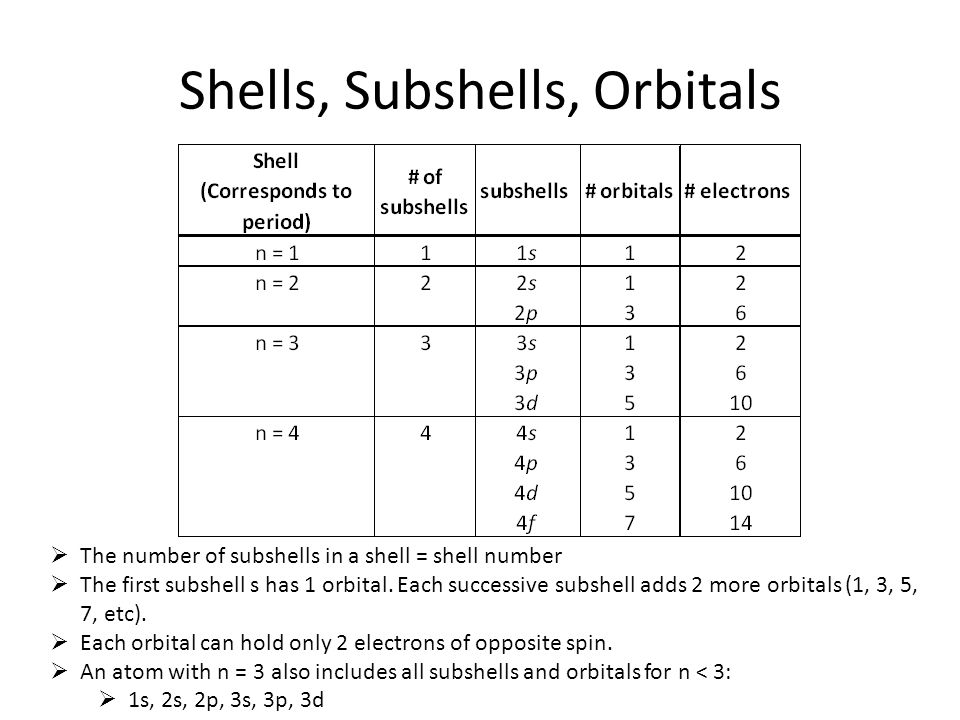
What Is Difference Between Shell And Orbital . Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Shell is the pathway followed by electrons around an atom’s nucleus. Within a shell (same n n), all. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Subshell is the pathway in which an electron. All electrons that have the same value for n n (the principle quantum number) are in the same shell.
from socratic.org
Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Within a shell (same n n), all. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Subshell is the pathway in which an electron. Shell is the pathway followed by electrons around an atom’s nucleus.
What is the difference between a shell and a subshell? Socratic
What Is Difference Between Shell And Orbital Shell is the pathway followed by electrons around an atom’s nucleus. Within a shell (same n n), all. Shell is the pathway followed by electrons around an atom’s nucleus. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Subshell is the pathway in which an electron.
From www.youtube.com
What is the difference between a shell, a subshell and an orbital What Is Difference Between Shell And Orbital This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Each shell is subdivided into subshells,. What Is Difference Between Shell And Orbital.
From www.vrogue.co
What Is The Difference Between Electron Shells And El vrogue.co What Is Difference Between Shell And Orbital Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Each shell is subdivided into subshells,. What Is Difference Between Shell And Orbital.
From byjus.com
what is the sequence of shell , subshell, orbitals , orbits , electrons What Is Difference Between Shell And Orbital Shell is the pathway followed by electrons around an atom’s nucleus. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Within a shell (same n n), all. Subshell is the pathway in which an electron. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. What Is Difference Between Shell And Orbital.
From www.youtube.com
Elements, Atoms, Shells, Subshells And Orbitals YouTube What Is Difference Between Shell And Orbital Shell is the pathway followed by electrons around an atom’s nucleus. Subshell is the pathway in which an electron. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. This video. What Is Difference Between Shell And Orbital.
From quizlet.com
Each _p_subshell can a maximum of __________ el Quizlet What Is Difference Between Shell And Orbital Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Shell is. What Is Difference Between Shell And Orbital.
From pediaa.com
Difference Between Shell Subshell and Orbital Definition, Structure What Is Difference Between Shell And Orbital Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Subshell is the pathway in which an electron. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Every shell has three p orbitals except for the first one (n = 1) the p orbitals. What Is Difference Between Shell And Orbital.
From www.vrogue.co
What Is The Difference Between Electron Shells And El vrogue.co What Is Difference Between Shell And Orbital This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Shell is the pathway followed by electrons around an atom’s nucleus. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. Each shell is subdivided into subshells, which are made up. What Is Difference Between Shell And Orbital.
From www.coscinecreative.com
What is the Difference in a Shell, Subshell and Orbital? — CoScine Creative What Is Difference Between Shell And Orbital Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where. What Is Difference Between Shell And Orbital.
From mungfali.com
Orbital Subshell Chart What Is Difference Between Shell And Orbital Within a shell (same n n), all. Shell is the pathway followed by electrons around an atom’s nucleus. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Shell refers to. What Is Difference Between Shell And Orbital.
From www.youtube.com
Shells, Subshells, and Orbitals l Understand the difference YouTube What Is Difference Between Shell And Orbital Shell is the pathway followed by electrons around an atom’s nucleus. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. Subshell is the pathway in which an electron. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. All electrons. What Is Difference Between Shell And Orbital.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent What Is Difference Between Shell And Orbital Subshell is the pathway in which an electron. Within a shell (same n n), all. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Shell is the pathway followed by. What Is Difference Between Shell And Orbital.
From www.youtube.com
Difference Between Orbits and Orbitals Chemistry YouTube What Is Difference Between Shell And Orbital Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Subshell is the pathway in which an electron. Every shell has three p orbitals except for the first one (n =. What Is Difference Between Shell And Orbital.
From www.youtube.com
ORBIT vs ORBITAL electrons 3min Quick short differences YouTube What Is Difference Between Shell And Orbital Shell is the pathway followed by electrons around an atom’s nucleus. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Within a shell (same n n), all. Subshell is the. What Is Difference Between Shell And Orbital.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills What Is Difference Between Shell And Orbital Within a shell (same n n), all. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles. What Is Difference Between Shell And Orbital.
From socratic.org
What is the difference between electron shells and electron orbitals What Is Difference Between Shell And Orbital Subshell is the pathway in which an electron. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Within a shell (same n n), all. All electrons that have the same. What Is Difference Between Shell And Orbital.
From socratic.org
What is the difference between a shell and a subshell? Socratic What Is Difference Between Shell And Orbital Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Shell. What Is Difference Between Shell And Orbital.
From www.youtube.com
What is the Difference between Shell and Subshell Chemistry Class 9 What Is Difference Between Shell And Orbital Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Shell. What Is Difference Between Shell And Orbital.
From www.pinterest.com
Atoms, shells ,Subshells and Orbitals Atom, Submarine, Shells What Is Difference Between Shell And Orbital This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Within a shell (same n n), all. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Subshell is the pathway in which an electron. Every shell has three p orbitals except for the first. What Is Difference Between Shell And Orbital.
From www.youtube.com
Structure of Atom Class 11 Chemistry Difference between orbit and What Is Difference Between Shell And Orbital Shell is the pathway followed by electrons around an atom’s nucleus. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Subshell is the pathway in which an electron. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Shell refers to an energy level in. What Is Difference Between Shell And Orbital.
From www.youtube.com
What is Difference between Shell, Sub shell and Orbital Structure What Is Difference Between Shell And Orbital Within a shell (same n n), all. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Shell refers to an energy level in an atom containing electrons, while an orbital. What Is Difference Between Shell And Orbital.
From mungfali.com
Orbital Subshell Chart What Is Difference Between Shell And Orbital Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. This. What Is Difference Between Shell And Orbital.
From www.youtube.com
What are shells,subshells and orbitals? Difference between shells What Is Difference Between Shell And Orbital Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. Within a shell (same n n), all. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles. What Is Difference Between Shell And Orbital.
From www.coscinecreative.com
What is the Difference in a Shell, Subshell and Orbital? — CoScine Creative What Is Difference Between Shell And Orbital Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Shell is the pathway followed by electrons around an atom’s nucleus. Within a shell (same n. What Is Difference Between Shell And Orbital.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is Difference Between Shell And Orbital Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. Subshell is the pathway in which an electron. This video explains the concepts of shells, subshells,. What Is Difference Between Shell And Orbital.
From www.vrogue.co
What Is The Difference Between Shell And Subshell Che vrogue.co What Is Difference Between Shell And Orbital Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Within. What Is Difference Between Shell And Orbital.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica What Is Difference Between Shell And Orbital Within a shell (same n n), all. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. Shell is the pathway followed by electrons around an. What Is Difference Between Shell And Orbital.
From www.youtube.com
Shells, Subshells, and Orbitals, Oh My! YouTube What Is Difference Between Shell And Orbital Shell is the pathway followed by electrons around an atom’s nucleus. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to. What Is Difference Between Shell And Orbital.
From www.vrogue.co
What Is The Difference Between Shell And Subshell Che vrogue.co What Is Difference Between Shell And Orbital Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular. What Is Difference Between Shell And Orbital.
From www.britannica.com
Sorbital physics Britannica What Is Difference Between Shell And Orbital Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Shell is the pathway followed by electrons around an atom’s nucleus. Each shell is subdivided into subshells, which are made up. What Is Difference Between Shell And Orbital.
From www.coscinecreative.com
What is the Difference in a Shell, Subshell and Orbital? — CoScine Creative What Is Difference Between Shell And Orbital Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Within a shell (same n n), all. Every shell has three p orbitals except for the first. What Is Difference Between Shell And Orbital.
From socratic.org
What is the difference between Shell (orbit) , Subshell and orbital What Is Difference Between Shell And Orbital Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Subshell is the pathway in which an electron. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Within a shell (same n n), all. This video explains the concepts of. What Is Difference Between Shell And Orbital.
From mungfali.com
Orbital Subshell Chart What Is Difference Between Shell And Orbital Subshell is the pathway in which an electron. Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Shell is the pathway followed by electrons around an atom’s nucleus. All electrons that have the same value for n n (the principle quantum number) are in the same shell. This video. What Is Difference Between Shell And Orbital.
From www.slideserve.com
PPT Chapter Three PowerPoint Presentation, free download ID5840496 What Is Difference Between Shell And Orbital Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. Subshell. What Is Difference Between Shell And Orbital.
From chem.libretexts.org
6.6 3D Representation of Orbitals Chemistry LibreTexts What Is Difference Between Shell And Orbital Every shell has three p orbitals except for the first one (n = 1) the p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another;. Subshell is the pathway in which an electron. Shell refers to an energy level in an atom containing electrons, while an. What Is Difference Between Shell And Orbital.
From projectopenletter.com
What Orbitals Are Used To Form The Bond Indicated In The Following What Is Difference Between Shell And Orbital Each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different angular. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely. All electrons that have the same value for n n (the principle quantum number) are in. What Is Difference Between Shell And Orbital.