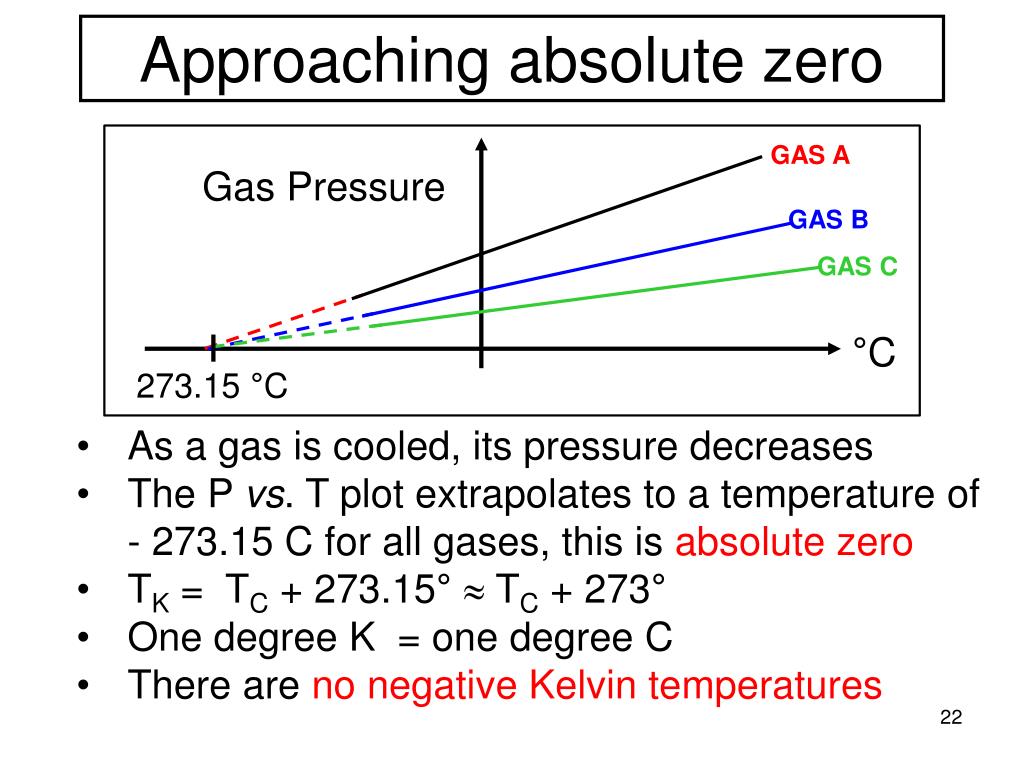
Absolute Zero Pressure Is Obtained . Absolute zero can be defined as the temperature at which matter does not move. At this temperature, particles in a substance have minimal. If you use the ideal gas law, and use 1 mole of gas in one liter and calculate accordingly using the equation p. This corresponds to zero kelvin, or minus 273.15 c. At absolute zero, even subatomic vibrations are. Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. Absolute pressure is the sum of gauge pressure and atmospheric pressure. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative.
from www.slideserve.com
This corresponds to zero kelvin, or minus 273.15 c. If you use the ideal gas law, and use 1 mole of gas in one liter and calculate accordingly using the equation p. Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. At this temperature, particles in a substance have minimal. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. Absolute zero can be defined as the temperature at which matter does not move. At absolute zero, even subatomic vibrations are. Absolute pressure is the sum of gauge pressure and atmospheric pressure.
PPT L 16 Thermodynamics1 PowerPoint Presentation, free download ID
Absolute Zero Pressure Is Obtained Absolute zero can be defined as the temperature at which matter does not move. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. This corresponds to zero kelvin, or minus 273.15 c. At absolute zero, even subatomic vibrations are. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. If you use the ideal gas law, and use 1 mole of gas in one liter and calculate accordingly using the equation p. At this temperature, particles in a substance have minimal. Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. Absolute pressure is the sum of gauge pressure and atmospheric pressure. Absolute zero can be defined as the temperature at which matter does not move.
From www.slideserve.com
PPT C H A P T E R 12 Temperature and Heat PowerPoint Presentation Absolute Zero Pressure Is Obtained If you use the ideal gas law, and use 1 mole of gas in one liter and calculate accordingly using the equation p. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. Absolute zero can be defined as the temperature at which matter does not move. This corresponds to zero kelvin, or. Absolute Zero Pressure Is Obtained.
From www.slideserve.com
PPT Properties of Fluids for Fluid Mechanics PowerPoint Presentation Absolute Zero Pressure Is Obtained Absolute pressure is the sum of gauge pressure and atmospheric pressure. Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. This corresponds to zero kelvin, or minus 273.15 c. At absolute zero, even subatomic vibrations are. For reasons we will explore later, in most. Absolute Zero Pressure Is Obtained.
From chemistryskills.com
Absolute Zero Chemistry Skills Absolute Zero Pressure Is Obtained Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. This corresponds to zero kelvin, or minus 273.15 c. At this temperature, particles in a substance have minimal.. Absolute Zero Pressure Is Obtained.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID6307179 Absolute Zero Pressure Is Obtained Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. At. Absolute Zero Pressure Is Obtained.
From www.slideserve.com
PPT AP Physics B PowerPoint Presentation, free download ID719065 Absolute Zero Pressure Is Obtained Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. If you use the ideal gas law, and use 1 mole of gas in one liter and calculate. Absolute Zero Pressure Is Obtained.
From www.chegg.com
Solved 1. Explain what is absolute zero pressure \& Absolute Zero Pressure Is Obtained For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. Absolute pressure is the sum of gauge pressure and atmospheric pressure. This corresponds to zero kelvin, or minus 273.15 c. If you use the ideal gas law, and use 1 mole of gas in one liter and calculate accordingly using the equation p.. Absolute Zero Pressure Is Obtained.
From www.youtube.com
Finding Absolute Zero Using a Graph YouTube Absolute Zero Pressure Is Obtained This corresponds to zero kelvin, or minus 273.15 c. At absolute zero, even subatomic vibrations are. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. At this temperature, particles in a substance have minimal. Absolute. Absolute Zero Pressure Is Obtained.
From www.slideserve.com
PPT 5.3b Thermal Physics Gases PowerPoint Presentation, free download Absolute Zero Pressure Is Obtained This corresponds to zero kelvin, or minus 273.15 c. Absolute pressure is the sum of gauge pressure and atmospheric pressure. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. At this temperature, particles in a. Absolute Zero Pressure Is Obtained.
From www.chegg.com
Lab Experiment Absolute Zero Plot absolute pressure Absolute Zero Pressure Is Obtained Absolute pressure is the sum of gauge pressure and atmospheric pressure. At absolute zero, even subatomic vibrations are. At this temperature, particles in a substance have minimal. This corresponds to zero kelvin, or minus 273.15 c. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. For reasons we will explore later, in. Absolute Zero Pressure Is Obtained.
From slideplayer.com
Chapter 2 FLUID STATICS. ppt download Absolute Zero Pressure Is Obtained This corresponds to zero kelvin, or minus 273.15 c. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. Absolute zero can be defined as the temperature at. Absolute Zero Pressure Is Obtained.
From twyfordphysicsy10.blogspot.com
GCSE Physics Gas pressure and absolute zero Absolute Zero Pressure Is Obtained Absolute pressure is the sum of gauge pressure and atmospheric pressure. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. At this temperature, particles in a substance have minimal. Absolute zero can be defined as the temperature at which matter does not move. For reasons we will explore later, in most cases. Absolute Zero Pressure Is Obtained.
From chart-studio.plotly.com
Absolute Zero Prediction Temperature (C) vs. Pressure (kPa Absolute Zero Pressure Is Obtained Absolute zero can be defined as the temperature at which matter does not move. Absolute pressure is the sum of gauge pressure and atmospheric pressure. Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. Absolute pressure is measured relative to absolute zero on the. Absolute Zero Pressure Is Obtained.
From www.slideserve.com
PPT Gas Law and Gas Behavior PowerPoint Presentation ID3183351 Absolute Zero Pressure Is Obtained Absolute pressure is the sum of gauge pressure and atmospheric pressure. Absolute zero can be defined as the temperature at which matter does not move. This corresponds to zero kelvin, or minus 273.15 c. At absolute zero, even subatomic vibrations are. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. Absolute zero. Absolute Zero Pressure Is Obtained.
From www.slideserve.com
PPT Chapter 2 BASIC CONCEPTS OF THERMODYNAMICS PowerPoint Absolute Zero Pressure Is Obtained At absolute zero, even subatomic vibrations are. At this temperature, particles in a substance have minimal. Absolute zero can be defined as the temperature at which matter does not move. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. Absolute pressure is the sum of gauge pressure and atmospheric pressure. This corresponds. Absolute Zero Pressure Is Obtained.
From www.slideserve.com
PPT This temperature has been called Absolute Zero . PowerPoint Absolute Zero Pressure Is Obtained Absolute pressure is the sum of gauge pressure and atmospheric pressure. Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. Absolute zero can be defined as the temperature at which matter does not move. At absolute zero, even subatomic vibrations are. For reasons we. Absolute Zero Pressure Is Obtained.
From stock.adobe.com
Types of Pressure Infographic Diagram including atmospheric absolute Absolute Zero Pressure Is Obtained If you use the ideal gas law, and use 1 mole of gas in one liter and calculate accordingly using the equation p. Absolute pressure is the sum of gauge pressure and atmospheric pressure. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. At absolute zero, even subatomic vibrations are. Absolute zero. Absolute Zero Pressure Is Obtained.
From www.slideserve.com
PPT Gas Temperature, Volume, and Pressure PowerPoint Presentation Absolute Zero Pressure Is Obtained At absolute zero, even subatomic vibrations are. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. Absolute pressure is measured relative to absolute zero on the pressure. Absolute Zero Pressure Is Obtained.
From quizzlistpygmalion.z13.web.core.windows.net
Facts About Absolute Zero Absolute Zero Pressure Is Obtained Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. Absolute zero can be defined as the temperature at which matter does not move. At absolute zero, even subatomic vibrations are. Absolute pressure is measured relative to absolute zero on the pressure scale, which is. Absolute Zero Pressure Is Obtained.
From twyfordphysicsy10.blogspot.com
GCSE Physics Gas pressure and absolute zero Absolute Zero Pressure Is Obtained Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. This corresponds to zero kelvin, or minus 273.15 c. Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. Absolute zero can be defined as the temperature at. Absolute Zero Pressure Is Obtained.
From blog.mensor.com
How to Zero Absolute Pressure Transducers and Sensors Absolute Zero Pressure Is Obtained At this temperature, particles in a substance have minimal. This corresponds to zero kelvin, or minus 273.15 c. Absolute pressure is the sum of gauge pressure and atmospheric pressure. If you use the ideal gas law, and use 1 mole of gas in one liter and calculate accordingly using the equation p. For reasons we will explore later, in most. Absolute Zero Pressure Is Obtained.
From www.astro.purdue.edu
Demos Department of Physics and Astronomy Purdue University Absolute Zero Pressure Is Obtained Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. If. Absolute Zero Pressure Is Obtained.
From www.youtube.com
Absolute Zero Pressure in Thermodynamics Pressure Reaches a value of Absolute Zero Pressure Is Obtained Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. At this temperature, particles in a substance have minimal. Absolute zero can be defined as the temperature at which matter does not move. For reasons we will explore later, in most cases the absolute pressure. Absolute Zero Pressure Is Obtained.
From chart-studio.plotly.com
Finding Absolute Zero by a Temperature vs Pressure Graph scatter Absolute Zero Pressure Is Obtained Absolute zero can be defined as the temperature at which matter does not move. Absolute pressure is the sum of gauge pressure and atmospheric pressure. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. This corresponds to zero kelvin, or minus 273.15 c. Absolute pressure is measured relative to absolute zero on. Absolute Zero Pressure Is Obtained.
From bet.yonsei.ac.kr
Types Of Pressure Infographic Diagram Including Atmospheric Absolute Absolute Zero Pressure Is Obtained For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. If you use the ideal gas law, and use 1 mole of gas in one liter and calculate accordingly using the equation p. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. Absolute zero is. Absolute Zero Pressure Is Obtained.
From www.slideserve.com
PPT Lecture N. 8 Thermodynamics (concepts, definitions, and basic Absolute Zero Pressure Is Obtained Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. If you use the ideal gas law, and use 1 mole of gas in one liter and calculate accordingly using the equation p. At absolute zero, even subatomic vibrations are. Absolute zero can be defined as the temperature at which matter does not. Absolute Zero Pressure Is Obtained.
From www.slideserve.com
PPT All You Need to Know About Pressure Measurement PowerPoint Absolute Zero Pressure Is Obtained If you use the ideal gas law, and use 1 mole of gas in one liter and calculate accordingly using the equation p. Absolute pressure is the sum of gauge pressure and atmospheric pressure. At absolute zero, even subatomic vibrations are. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. Absolute zero. Absolute Zero Pressure Is Obtained.
From www.expii.com
Absolute Zero — Definition & Importance Expii Absolute Zero Pressure Is Obtained Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. Absolute zero can be defined as the temperature at which matter does not move. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. If you use the. Absolute Zero Pressure Is Obtained.
From www.slideserve.com
PPT L 16 Thermodynamics1 PowerPoint Presentation, free download ID Absolute Zero Pressure Is Obtained Absolute zero can be defined as the temperature at which matter does not move. At this temperature, particles in a substance have minimal. Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale. This corresponds to zero kelvin, or minus 273.15 c. Absolute pressure is. Absolute Zero Pressure Is Obtained.
From www.pressure-sensors.co.uk
What's the Difference Between Gauge and Absolute Pressure Absolute Zero Pressure Is Obtained Absolute pressure is the sum of gauge pressure and atmospheric pressure. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. This corresponds to zero kelvin, or minus 273.15 c. At absolute zero, even subatomic vibrations are. Absolute zero is defined as the point where no more heat can be removed from a. Absolute Zero Pressure Is Obtained.
From spiff.rit.edu
Physics 212 Lecture Absolute Zero Pressure Is Obtained At absolute zero, even subatomic vibrations are. This corresponds to zero kelvin, or minus 273.15 c. If you use the ideal gas law, and use 1 mole of gas in one liter and calculate accordingly using the equation p. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. For reasons we will. Absolute Zero Pressure Is Obtained.
From www.youtube.com
How to use an Equation to find Absolute Zero on an Excel Pressure Absolute Zero Pressure Is Obtained Absolute pressure is the sum of gauge pressure and atmospheric pressure. At absolute zero, even subatomic vibrations are. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. If you use the ideal gas law, and use 1 mole of gas in one liter and calculate accordingly using the equation p. Absolute zero. Absolute Zero Pressure Is Obtained.
From japaneseclass.jp
Images of Absolute Zero JapaneseClass.jp Absolute Zero Pressure Is Obtained At absolute zero, even subatomic vibrations are. Absolute pressure is the sum of gauge pressure and atmospheric pressure. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. Absolute zero is defined as the point where no more heat can be removed from a system, according to the absolute or thermodynamic temperature scale.. Absolute Zero Pressure Is Obtained.
From www.chegg.com
Solved Example 61 Determination of absolute zero Absolute Zero Pressure Is Obtained At absolute zero, even subatomic vibrations are. Absolute pressure is the sum of gauge pressure and atmospheric pressure. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. If you use the ideal gas law, and use 1 mole of gas in one liter and calculate accordingly using the equation p. Absolute pressure. Absolute Zero Pressure Is Obtained.
From www.youtube.com
Absolute Zero and Pressure YouTube Absolute Zero Pressure Is Obtained Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. At absolute zero, even subatomic vibrations are. For reasons we will explore later, in most cases the absolute pressure in fluids cannot be negative. Absolute pressure is the sum of gauge pressure and atmospheric pressure. If you use the ideal gas law, and. Absolute Zero Pressure Is Obtained.
From slideplayer.com
Tro's Introductory Chemistry, Chapter ppt download Absolute Zero Pressure Is Obtained At absolute zero, even subatomic vibrations are. Absolute zero can be defined as the temperature at which matter does not move. At this temperature, particles in a substance have minimal. Absolute pressure is measured relative to absolute zero on the pressure scale, which is a perfect vacuum. Absolute pressure is the sum of gauge pressure and atmospheric pressure. This corresponds. Absolute Zero Pressure Is Obtained.