Standard Reduction Potential Definition With Example . It is expressed in volts (v) and. A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced.
from www.slideserve.com
A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. It is expressed in volts (v) and.
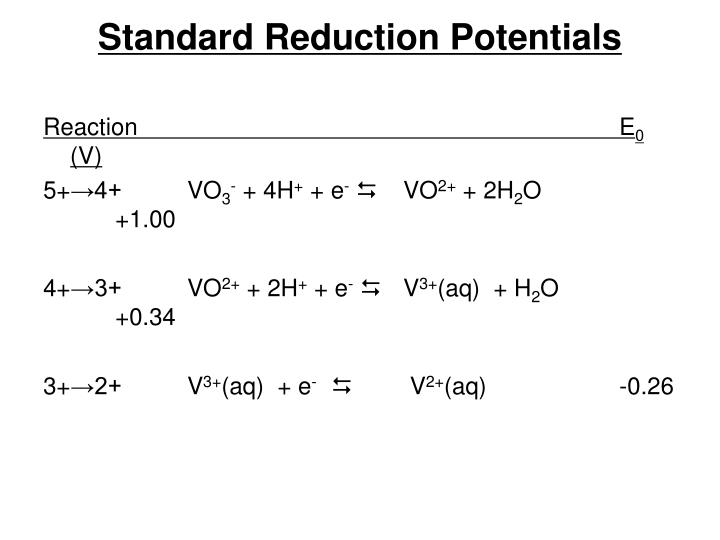
PPT The Oxidation States of Vanadium PowerPoint Presentation ID4176570
Standard Reduction Potential Definition With Example A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. It is expressed in volts (v) and. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Definition With Example Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. It is expressed in volts (v) and. Standard reduction potential is a measure of the tendency of a chemical species to. Standard Reduction Potential Definition With Example.
From www.slideserve.com
PPT The Oxidation States of Vanadium PowerPoint Presentation ID4176570 Standard Reduction Potential Definition With Example The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical. Standard Reduction Potential Definition With Example.
From testbook.com
Reduction Potential Measurement, Half Cells, Differences & More Standard Reduction Potential Definition With Example Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. It is expressed in volts (v) and. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency. Standard Reduction Potential Definition With Example.
From ch302.cm.utexas.edu
Standard Reduction Potentials Standard Reduction Potential Definition With Example It is expressed in volts (v) and. A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction potential is the tendency for a chemical species to be. Standard Reduction Potential Definition With Example.
From www.youtube.com
Difference between Oxidation potential and Reduction potential Standard Reduction Potential Definition With Example A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. It is. Standard Reduction Potential Definition With Example.
From www.slideserve.com
PPT Oxidation and Reduction PowerPoint Presentation, free download Standard Reduction Potential Definition With Example It is expressed in volts (v) and. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a. Standard Reduction Potential Definition With Example.
From www.youtube.com
Standard Reduction Potential YouTube Standard Reduction Potential Definition With Example Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. Use standard reduction potentials to determine. Standard Reduction Potential Definition With Example.
From chem.libretexts.org
Extra Credit 24 Chemistry LibreTexts Standard Reduction Potential Definition With Example It is expressed in volts (v) and. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. The standard reduction potential can be determined by subtracting the standard reduction. Standard Reduction Potential Definition With Example.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Definition With Example Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. It is expressed in volts (v) and. A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured. Standard Reduction Potential Definition With Example.
From www.slideserve.com
PPT Product stabilizations in hydrolysis PowerPoint Presentation Standard Reduction Potential Definition With Example The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. It is expressed in volts (v) and. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. A serial arrangement of metallic elements or ions. Standard Reduction Potential Definition With Example.
From www.scribd.com
Table of Standard Reduction Potential PDF Standard Reduction Potential Definition With Example Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. It is expressed in volts (v) and. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency. Standard Reduction Potential Definition With Example.
From www.researchgate.net
Standard reduction potentials versus SHE; data from references [89,91 Standard Reduction Potential Definition With Example A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. It is expressed in volts (v) and. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The. Standard Reduction Potential Definition With Example.
From www.flinnsci.ca
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Definition With Example Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard. Standard Reduction Potential Definition With Example.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Definition With Example It is expressed in volts (v) and. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. The standard reduction potential is the tendency for a chemical species to be reduced, and is. Standard Reduction Potential Definition With Example.
From f15.beauty
Standard Reduction Potential Table Standard Reduction Potential Definition With Example Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. It is expressed in volts (v). Standard Reduction Potential Definition With Example.
From dxoqcxjmj.blob.core.windows.net
Standard Reduction Potential Example at Toni Officer blog Standard Reduction Potential Definition With Example The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The standard reduction. Standard Reduction Potential Definition With Example.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Definition With Example The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard. Standard Reduction Potential Definition With Example.
From www.albertgural.com
Equations Albert Gural Standard Reduction Potential Definition With Example It is expressed in volts (v) and. A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices.. Standard Reduction Potential Definition With Example.
From klaecwhyz.blob.core.windows.net
Standard Reduction Potential Ph at Shane Marlowe blog Standard Reduction Potential Definition With Example Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. It. Standard Reduction Potential Definition With Example.
From app.pandai.org
Determine oxidising agent and reducing agent based on value of standard Standard Reduction Potential Definition With Example The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. It is expressed in volts (v) and. The standard reduction potential is the tendency for a chemical species to. Standard Reduction Potential Definition With Example.
From www.youtube.com
Standard reduction potentials Redox reactions and electrochemistry Standard Reduction Potential Definition With Example Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction. Standard Reduction Potential Definition With Example.
From www.chegg.com
Solved Use the standard reduction potentials located in the Standard Reduction Potential Definition With Example Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. It is expressed in volts (v) and. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. A serial arrangement of metallic elements or ions based on their. Standard Reduction Potential Definition With Example.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Definition With Example The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction potential can be determined by subtracting the standard reduction potential for the. Standard Reduction Potential Definition With Example.
From oneclass.com
OneClass Given the following halfreactions and their respective Standard Reduction Potential Definition With Example Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. A. Standard Reduction Potential Definition With Example.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Definition With Example Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. It is expressed in volts (v) and. A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions.. Standard Reduction Potential Definition With Example.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Definition With Example Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. Standard reduction potential is a measure of the. Standard Reduction Potential Definition With Example.
From www.pinterest.co.kr
Standard Reduction Potential (E) when given two half reactions and Standard Reduction Potential Definition With Example The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Standard reduction potential. Standard Reduction Potential Definition With Example.
From askfilo.com
Standard reduction potential of half reactions are given below Au3+(aq)+3.. Standard Reduction Potential Definition With Example It is expressed in volts (v) and. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. The standard reduction potential is the tendency for a chemical species to be reduced, and is. Standard Reduction Potential Definition With Example.
From www.pinterest.ca
Unit 7 Nuclear and Teaching chemistry, Chemistry basics Standard Reduction Potential Definition With Example The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard. Standard Reduction Potential Definition With Example.
From www.scribd.com
Standard Reduction Potentials Data Extended pdf Standard Reduction Potential Definition With Example The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. Use standard reduction. Standard Reduction Potential Definition With Example.
From chemistrynotes.com
Cell Potential Calculations and Line Notation Standard Reduction Potential Definition With Example Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. It is expressed in volts (v). Standard Reduction Potential Definition With Example.
From chemistrytalk.org
Standard Reduction Potentials made easy ChemTalk Standard Reduction Potential Definition With Example Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. It is expressed in volts (v) and. The standard reduction potential can be determined by subtracting the standard reduction. Standard Reduction Potential Definition With Example.
From www.chegg.com
Solved Given these standard reduction potentials at 25 Standard Reduction Potential Definition With Example A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. It is expressed in volts (v) and. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in. Standard Reduction Potential Definition With Example.
From www.reddit.com
Standard Reduction Potential r/Mcat Standard Reduction Potential Definition With Example Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. Standard Reduction Potential Definition With Example.
From www.numerade.com
SOLVED Standard Reduction Potentials at 25^∘C^* Copyright (c) The Standard Reduction Potential Definition With Example A serial arrangement of metallic elements or ions based on their electrode potentials obtained under specific conditions. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. The standard reduction. Standard Reduction Potential Definition With Example.