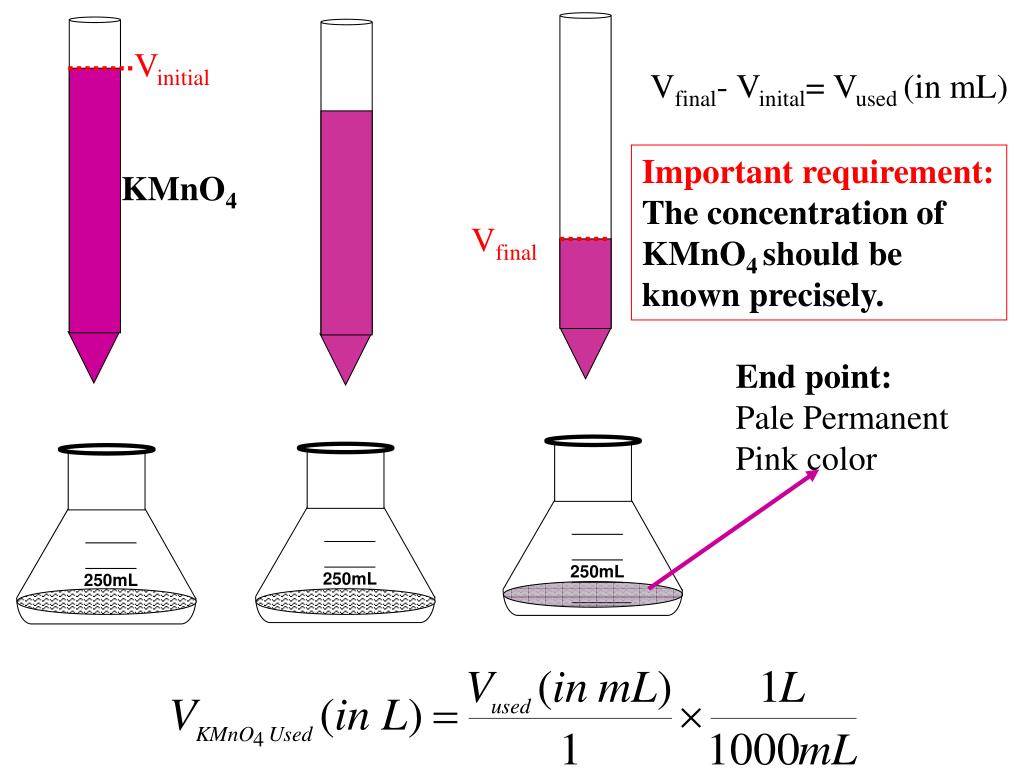
Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of . Ensure that the titration is performed in an acidic medium because kmno4 is most stable in acid. Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. In the oxidation reactions of k m n o 4, in acidic medium, only h 2 s o 4 is used to product acidic medium but not hcl(or ) h n o 3 because a hcl reacts with k m n o 4 and. However, the hcl is oxidized when a few drops of. Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is = 6. → mn2+ + 4h 2o. When ferrous ammonium sulfate solution is titrated against potassium permanganate in the presence of acidic medium by sulfuric acid. The oxidising action of kmno in the acidic medium can be represented by the following. K m n o 4. Reason for hcl is not used in titration: A kmno4 solution can be standarised by titration against as2o3 (s). Typically, we add hcl to the kmno 4 solution to make it acidic. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq)
from www.slideserve.com
Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is = 6. → mn2+ + 4h 2o. Reason for hcl is not used in titration: In the oxidation reactions of k m n o 4, in acidic medium, only h 2 s o 4 is used to product acidic medium but not hcl(or ) h n o 3 because a hcl reacts with k m n o 4 and. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) Ensure that the titration is performed in an acidic medium because kmno4 is most stable in acid. The oxidising action of kmno in the acidic medium can be represented by the following. Typically, we add hcl to the kmno 4 solution to make it acidic. A kmno4 solution can be standarised by titration against as2o3 (s).
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911
Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is = 6. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) When ferrous ammonium sulfate solution is titrated against potassium permanganate in the presence of acidic medium by sulfuric acid. The oxidising action of kmno in the acidic medium can be represented by the following. Reason for hcl is not used in titration: However, the hcl is oxidized when a few drops of. Typically, we add hcl to the kmno 4 solution to make it acidic. → mn2+ + 4h 2o. In the oxidation reactions of k m n o 4, in acidic medium, only h 2 s o 4 is used to product acidic medium but not hcl(or ) h n o 3 because a hcl reacts with k m n o 4 and. A kmno4 solution can be standarised by titration against as2o3 (s). Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. Ensure that the titration is performed in an acidic medium because kmno4 is most stable in acid. K m n o 4.
From www.youtube.com
KMnO4 in Acid, Base and Neutral Medium Oxidation State Equivalent Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Typically, we add hcl to the kmno 4 solution to make it acidic. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of When ferrous ammonium sulfate solution is titrated against potassium permanganate in the presence of acidic medium by sulfuric acid. Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. Reason for hcl is not used in titration: Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
71 Molecular weight of KMnO4 in acidic medium and neutral medium will Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Ensure that the titration is performed in an acidic medium because kmno4 is most stable in acid. Reason for hcl is not used in titration: Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) However, the. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.toppr.com
The number of mole of KMnO4 that will be needed to react with one mole Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is = 6. Ensure that the titration is performed in an acidic medium because kmno4 is most stable in acid. However, the hcl is oxidized when a few drops of. In the oxidation reactions of k m n o 4, in acidic medium,. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. In the oxidation reactions of k m n o 4, in acidic medium, only h 2 s o 4 is used to product acidic medium but not hcl(or ) h n o 3 because a hcl reacts with k m. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.studypool.com
SOLUTION Titration mohr s salt vs kmno4 1 Studypool Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of The oxidising action of kmno in the acidic medium can be represented by the following. Reason for hcl is not used in titration: K m n o 4. Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. Ensure that the titration is performed in an acidic medium because kmno4. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
the moles of KMnO4 require to oxidise 100ml of 1volume H2O2 in acidic Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of A kmno4 solution can be standarised by titration against as2o3 (s). Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. Reason for hcl is not used in titration: → mn2+ + 4h 2o. However, the hcl is oxidized when a few drops of. Typically, we add hcl to the. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.toppr.com
KMnO4 acts as an oxidizing agent in acidic medium. The number of moles Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of The oxidising action of kmno in the acidic medium can be represented by the following. K m n o 4. When ferrous ammonium sulfate solution is titrated against potassium permanganate in the presence of acidic medium by sulfuric acid. Ensure that the titration is performed in an acidic medium because kmno4 is most stable in acid. Spin only value of. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.mutualgreget.com
What Is The Molar Mass Of Kmno4 mutualgreget Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Reason for hcl is not used in titration: In the oxidation reactions of k m n o 4, in acidic medium, only h 2 s o 4 is used to product acidic medium but not hcl(or ) h n o 3 because a hcl reacts with k m n o 4 and. Ensure that the titration is performed in an. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.slideserve.com
PPT REDOX TITRATIONS PowerPoint Presentation ID2670469 Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of However, the hcl is oxidized when a few drops of. Reason for hcl is not used in titration: K m n o 4. Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is = 6. A kmno4 solution can be standarised by titration against as2o3 (s). A 0.1156 g sample of as2o3. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.toppr.com
How many moles of KMnO4 are required to oxidize one mole of SnCl2 in Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of In the oxidation reactions of k m n o 4, in acidic medium, only h 2 s o 4 is used to product acidic medium but not hcl(or ) h n o 3 because a hcl reacts with k m n o 4 and. → mn2+ + 4h 2o. However, the hcl is oxidized when a few drops of. A. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.youtube.com
How to Balance Acidic KMnO4 + H2S( Half Reaction Method in acidic Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of However, the hcl is oxidized when a few drops of. → mn2+ + 4h 2o. When ferrous ammonium sulfate solution is titrated against potassium permanganate in the presence of acidic medium by sulfuric acid. Ensure that the titration is performed in an acidic medium because kmno4 is most stable in acid. The oxidising action of kmno in the acidic medium. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From askfilo.com
When 10 mL of an aqueous solution of KMnO4 was titrated in acidic medium.. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Ensure that the titration is performed in an acidic medium because kmno4 is most stable in acid. When ferrous ammonium sulfate solution is titrated against potassium permanganate in the presence of acidic medium by sulfuric acid. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) Reason for hcl is not used in titration: Typically, we add. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.youtube.com
Titration Of Kmno4 Vs Oxalic Acid The Ultimate Guide Titration Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is = 6. K m n o 4. The oxidising action of kmno in the acidic medium can be represented by the following.. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.youtube.com
KMnO4 Reactions in Acidic Medium Class 12th How to Balance Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of In the oxidation reactions of k m n o 4, in acidic medium, only h 2 s o 4 is used to product acidic medium but not hcl(or ) h n o 3 because a hcl reacts with k m n o 4 and. However, the hcl is oxidized when a few drops of. A 0.1156 g sample of as2o3. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
100 mL of 1 M KMnO4 oxidised 100 mL H2O2 in acidic. Calculate the Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. K m n o 4. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) When ferrous ammonium sulfate solution is titrated against potassium permanganate in the presence of acidic medium by sulfuric acid. The oxidising action. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.toppr.com
Acidified KMnO4 oxidises oxalic acid to CO2 . What is the volume (in Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Typically, we add hcl to the kmno 4 solution to make it acidic. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) However, the hcl is oxidized when a few drops of. Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. K m n o. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
8 gm sample of KMnO4 is rected in acidic medium with 100ml of 10 valume Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Ensure that the titration is performed in an acidic medium because kmno4 is most stable in acid. Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is = 6. Typically, we add hcl to the kmno 4 solution to make it acidic. → mn2+ + 4h 2o. In the oxidation reactions of. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.youtube.com
Why KMnO4 is a strong oxidizing agent in acidic medium?? YouTube Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of The oxidising action of kmno in the acidic medium can be represented by the following. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. Typically, we add hcl to the kmno 4 solution to make it. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.toppr.com
1 mole of equimolar mixture of ferric oxalate and ferrous oxalate will Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of → mn2+ + 4h 2o. K m n o 4. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) Reason for hcl is not used in titration: Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is = 6. The oxidising action of kmno in the acidic medium. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
Calculate the number of moles of KMNO4 required to oxidise one mole of Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Ensure that the titration is performed in an acidic medium because kmno4 is most stable in acid. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) A kmno4 solution can be standarised by titration against as2o3 (s). Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is =. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
One mole of KMnO4 is used for complete oxidation of FeSO4, FeC2O4 and Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) Ensure that the titration is performed in an acidic medium because kmno4 is most stable in acid. Typically, we add hcl to the kmno 4 solution to make it acidic. Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.youtube.com
titration of kmno4 with mohr's salt class 12 determine the Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Reason for hcl is not used in titration: → mn2+ + 4h 2o. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) A kmno4 solution can be standarised by titration against as2o3 (s). Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is = 6. Typically, we add. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
10. The number of moles of KMnO4 that will be needed to react Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) → mn2+ + 4h 2o. Typically, we add hcl to the kmno 4 solution to make it acidic. Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is = 6. Ensure that the titration is performed in an acidic. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
The weight of KMnO4 that can oxidise 100 ml of 0.2 M oxalic acid in Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of When ferrous ammonium sulfate solution is titrated against potassium permanganate in the presence of acidic medium by sulfuric acid. However, the hcl is oxidized when a few drops of. Typically, we add hcl to the kmno 4 solution to make it acidic. Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.micoope.com.gt
Reactions Of KMnO4 Important Oxidising Reactions Of, 57 OFF Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of In the oxidation reactions of k m n o 4, in acidic medium, only h 2 s o 4 is used to product acidic medium but not hcl(or ) h n o 3 because a hcl reacts with k m n o 4 and. A kmno4 solution can be standarised by titration against as2o3 (s). Typically, we add hcl to. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
Increasing order of oxidising power of KMnO4 in different medium(acidic Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) A kmno4 solution can be standarised by titration against as2o3 (s). Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Reason for hcl is not used in titration: In the oxidation reactions of k m n o 4, in acidic medium, only h 2 s o 4 is used to product acidic medium but not hcl(or ) h n o 3 because a hcl reacts with k m n o 4 and. Typically, we add hcl to the kmno 4. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.numerade.com
SOLVEDRedox Titrationi Acidic Medium Puch Slider Upto Add Volume of Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. → mn2+ + 4h 2o. Ensure that the titration is performed in an acidic medium because kmno4 is most stable in acid. K m n o 4. However, the hcl is oxidized when a few drops of. Typically, we add. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
0.2 g sample of H2O2 required 10 ml of 1N KMnO4 for complete reaction Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of K m n o 4. In the oxidation reactions of k m n o 4, in acidic medium, only h 2 s o 4 is used to product acidic medium but not hcl(or ) h n o 3 because a hcl reacts with k m n o 4 and. Reason for hcl is not used in titration: Ensure that the. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From www.numerade.com
SOLVED KMnO4, can be standardized in Potassium permanganate, acidic Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) Reason for hcl is not used in titration: Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is = 6. A kmno4 solution can be standarised by titration against as2o3 (s). K m n o 4. The oxidising action. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is = 6. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) K m n o 4. A kmno4 solution can be standarised by titration against as2o3 (s). When ferrous ammonium sulfate solution is titrated against potassium permanganate in. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
Find the number of moles of H2O2 required to completely react with Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of The oxidising action of kmno in the acidic medium can be represented by the following. Spin only value of manganese product fromed during titration of \(kmno_4\) aganist oxalic acid in acidic medium is = 6. Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. A 0.1156 g sample of. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
5.The number of moles of ferrous oxalate oxidised by one mole of kmno4 Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Typically, we add hcl to the kmno 4 solution to make it acidic. A 0.1156 g sample of as2o3 requires 27.08 ml of the kmno4 (aq) Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. A kmno4 solution can be standarised by titration against as2o3 (s). K m n. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.
From byjus.com
In Acidic medium moles of KMnO4 required to oxidise 1.5 moles of Cu2S Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of Ensure that the titration is performed in an acidic medium because kmno4 is most stable in acid. Typically, we add hcl to the kmno 4 solution to make it acidic. Equivalent weight of k m n o 4 in acidic medium in m/5 and in strongly alkaline medium in m/3. A kmno4 solution can be standarised by titration against as2o3. Titration Of Kmno4 In Acidic Medium Is Satisfactory In Presence Of.