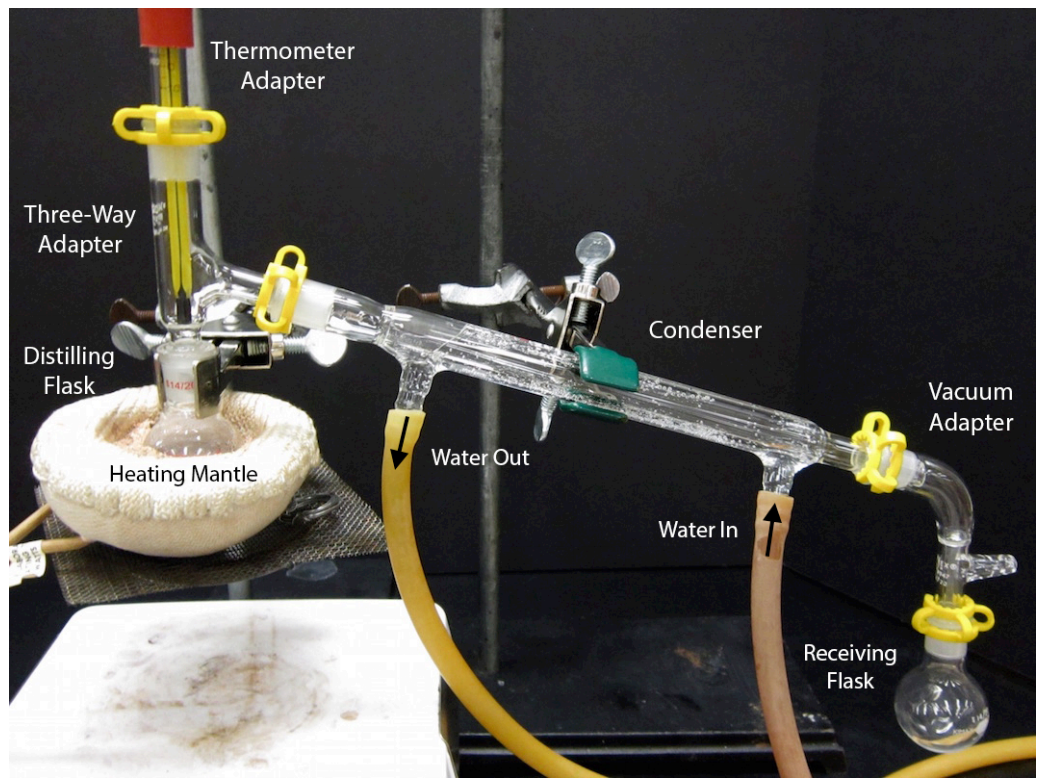
Use Of Distillation Thermometer . When different compounds in a. Apply the heat source to the distilling flask. There are two varieties of. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. Record the temperature where liquid is actively distilling and thermometer bulb is immersed. A distillation flask with a thermometer is placed in a heating mantle and is connected to a condenser. You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see possible compounds. Distillation setup is shown in figure 1. Collect distillate at a rate of 1 drop per second. The tubes on the condenser are attached to a water source,. Distillation is an extremely useful technique that is used to purify reagents and separate crude product mixtures. In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just as it condenses. Turn on the condenser water. To purify a mixture of two liquids using distillation.
from chem.libretexts.org
When different compounds in a. Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop per second. There are two varieties of. You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see possible compounds. A distillation flask with a thermometer is placed in a heating mantle and is connected to a condenser. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. To purify a mixture of two liquids using distillation. Distillation setup is shown in figure 1. Record the temperature where liquid is actively distilling and thermometer bulb is immersed.
5.2C StepbyStep Procedures Chemistry LibreTexts
Use Of Distillation Thermometer Distillation is an extremely useful technique that is used to purify reagents and separate crude product mixtures. The tubes on the condenser are attached to a water source,. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. When different compounds in a. Apply the heat source to the distilling flask. Distillation is an extremely useful technique that is used to purify reagents and separate crude product mixtures. To purify a mixture of two liquids using distillation. There are two varieties of. Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Turn on the condenser water. A distillation flask with a thermometer is placed in a heating mantle and is connected to a condenser. You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see possible compounds. Collect distillate at a rate of 1 drop per second. Distillation setup is shown in figure 1. In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just as it condenses.
From www.slideserve.com
PPT Experiment 6 Simple and Fractional Distillation PowerPoint Use Of Distillation Thermometer In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just as it condenses. You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see possible compounds. It consists of a flask containing the liquid to be distilled, an. Use Of Distillation Thermometer.
From www.slideserve.com
PPT Boiling Points Distillations PowerPoint Presentation, free Use Of Distillation Thermometer The tubes on the condenser are attached to a water source,. Distillation setup is shown in figure 1. There are two varieties of. In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just as it condenses. You will receive a vial containing 11.20 g of a mixture of two compounds. Use Of Distillation Thermometer.
From www.britannica.com
distillation summary Britannica Use Of Distillation Thermometer Record the temperature where liquid is actively distilling and thermometer bulb is immersed. When different compounds in a. In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just as it condenses. Apply the heat source to the distilling flask. Distillation setup is shown in figure 1. Turn on the condenser. Use Of Distillation Thermometer.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Use Of Distillation Thermometer Record the temperature where liquid is actively distilling and thermometer bulb is immersed. In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just as it condenses. You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see possible. Use Of Distillation Thermometer.
From www.researchgate.net
The distillation setup (1 thermometer, 2—still head, 3—round Use Of Distillation Thermometer Turn on the condenser water. Apply the heat source to the distilling flask. A distillation flask with a thermometer is placed in a heating mantle and is connected to a condenser. There are two varieties of. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. Distillation setup is shown. Use Of Distillation Thermometer.
From www.shipit2you.com
AlAmbik Thermometer for distillation, Size S, sensor 4 cm, Industry Use Of Distillation Thermometer The tubes on the condenser are attached to a water source,. Distillation is an extremely useful technique that is used to purify reagents and separate crude product mixtures. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. You will receive a vial containing 11.20 g of a mixture of. Use Of Distillation Thermometer.
From allcopper.pt
thermometer bimetallico distillation Use Of Distillation Thermometer To purify a mixture of two liquids using distillation. Distillation setup is shown in figure 1. When different compounds in a. There are two varieties of. Distillation is an extremely useful technique that is used to purify reagents and separate crude product mixtures. In the top of this connector is the thermometer, which is used to read the temperature of. Use Of Distillation Thermometer.
From www.slideserve.com
PPT Simple Distillation PowerPoint Presentation, free download ID Use Of Distillation Thermometer A distillation flask with a thermometer is placed in a heating mantle and is connected to a condenser. In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just as it condenses. Distillation is an extremely useful technique that is used to purify reagents and separate crude product mixtures. There are. Use Of Distillation Thermometer.
From www.vedantu.com
Distillation What Is Distillation? Process and Uses of Distillation Use Of Distillation Thermometer You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see possible compounds. To purify a mixture of two liquids using distillation. Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop per second. Distillation setup is shown in figure 1. When. Use Of Distillation Thermometer.
From abronexports.com
Glass distillation set 500ml flask round bottom condenser bend quick Use Of Distillation Thermometer It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. A distillation flask with a thermometer is placed in a heating mantle and is connected to a condenser. The tubes on the condenser are attached to a water source,. Distillation setup is shown in figure 1. Turn on the condenser. Use Of Distillation Thermometer.
From www.fishersci.se
Lenz Glass Distillation Thermometer Temp. Range Use Of Distillation Thermometer Distillation setup is shown in figure 1. Distillation is an extremely useful technique that is used to purify reagents and separate crude product mixtures. Record the temperature where liquid is actively distilling and thermometer bulb is immersed. When different compounds in a. The tubes on the condenser are attached to a water source,. To purify a mixture of two liquids. Use Of Distillation Thermometer.
From www.slideserve.com
PPT Methods of Purification PowerPoint Presentation, free download Use Of Distillation Thermometer To purify a mixture of two liquids using distillation. Turn on the condenser water. Apply the heat source to the distilling flask. A distillation flask with a thermometer is placed in a heating mantle and is connected to a condenser. In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just. Use Of Distillation Thermometer.
From www.vedantu.com
Uses of Distillation Learn Important Terms and Concepts Use Of Distillation Thermometer Turn on the condenser water. Apply the heat source to the distilling flask. Distillation is an extremely useful technique that is used to purify reagents and separate crude product mixtures. In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just as it condenses. There are two varieties of. To purify. Use Of Distillation Thermometer.
From chem.libretexts.org
5.3D StepbyStep Procedures for Fractional Distillation Chemistry Use Of Distillation Thermometer Collect distillate at a rate of 1 drop per second. Distillation setup is shown in figure 1. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. The tubes on the condenser are attached to a water source,. In the top of this connector is the thermometer, which is used. Use Of Distillation Thermometer.
From chem.libretexts.org
5.2C StepbyStep Procedures Chemistry LibreTexts Use Of Distillation Thermometer In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just as it condenses. Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Distillation setup is shown in figure 1. Turn on the condenser water. To purify a mixture of two liquids using distillation. When different compounds. Use Of Distillation Thermometer.
From www.numerade.com
In a distillation setup, the thermometer placement is extremely Use Of Distillation Thermometer To purify a mixture of two liquids using distillation. When different compounds in a. Distillation setup is shown in figure 1. The tubes on the condenser are attached to a water source,. Distillation is an extremely useful technique that is used to purify reagents and separate crude product mixtures. Record the temperature where liquid is actively distilling and thermometer bulb. Use Of Distillation Thermometer.
From milehidistilling.com
Adjustable Stainless Dial Thermometer For Distilling Use Of Distillation Thermometer Turn on the condenser water. Distillation setup is shown in figure 1. Collect distillate at a rate of 1 drop per second. To purify a mixture of two liquids using distillation. The tubes on the condenser are attached to a water source,. Apply the heat source to the distilling flask. In the top of this connector is the thermometer, which. Use Of Distillation Thermometer.
From mavink.com
Diagram Of Distillation Apparatus Use Of Distillation Thermometer You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see possible compounds. There are two varieties of. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. To purify a mixture of two liquids using distillation. Apply. Use Of Distillation Thermometer.
From www.bluebottlegin.gg
The Importance of the Thermometer Blue Bottle Gin Distillation Use Of Distillation Thermometer Record the temperature where liquid is actively distilling and thermometer bulb is immersed. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. When different compounds in a. You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c. Use Of Distillation Thermometer.
From www.pinterest.com
Figure 1 Fractional distillation apparatus using a Liebig condenser Use Of Distillation Thermometer Apply the heat source to the distilling flask. The tubes on the condenser are attached to a water source,. Collect distillate at a rate of 1 drop per second. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. In the top of this connector is the thermometer, which is. Use Of Distillation Thermometer.
From slidetodoc.com
Distillation Today you will learn About distillation Distillation Use Of Distillation Thermometer Turn on the condenser water. You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see possible compounds. Distillation is an extremely useful technique that is used to purify reagents and separate crude product mixtures. Collect distillate at a rate of 1 drop per second. To purify a. Use Of Distillation Thermometer.
From www.vedantu.com
Distillation What Is Distillation? Process and Uses of Distillation Use Of Distillation Thermometer Distillation setup is shown in figure 1. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. Collect distillate at a rate of 1 drop per second. You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see. Use Of Distillation Thermometer.
From www.pinterest.co.uk
Distillation Apparatus Diagram with full process and lab tools Use Of Distillation Thermometer A distillation flask with a thermometer is placed in a heating mantle and is connected to a condenser. Turn on the condenser water. The tubes on the condenser are attached to a water source,. There are two varieties of. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. You. Use Of Distillation Thermometer.
From www.researchgate.net
Schéma du montage de distillation 1,2 et 3 Support ; 4 thermomètre Use Of Distillation Thermometer A distillation flask with a thermometer is placed in a heating mantle and is connected to a condenser. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just as it. Use Of Distillation Thermometer.
From www.umsl.edu
Distillation Use Of Distillation Thermometer Distillation setup is shown in figure 1. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect. A distillation flask with a thermometer is placed in a heating mantle and is connected to a condenser. Turn on the condenser water. You will receive a vial containing 11.20 g of a. Use Of Distillation Thermometer.
From byjus.com
Fractional Distillation Detailed Explanation Along With Diagrams Use Of Distillation Thermometer Collect distillate at a rate of 1 drop per second. Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Distillation setup is shown in figure 1. You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see possible compounds. Apply the heat source to. Use Of Distillation Thermometer.
From www.istockphoto.com
Science Experiment With Distillation Stock Illustration Download Use Of Distillation Thermometer You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see possible compounds. There are two varieties of. Turn on the condenser water. When different compounds in a. Distillation setup is shown in figure 1. A distillation flask with a thermometer is placed in a heating mantle and. Use Of Distillation Thermometer.
From allcopper.pt
thermometer bimetallico distillation Use Of Distillation Thermometer The tubes on the condenser are attached to a water source,. There are two varieties of. Apply the heat source to the distilling flask. You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see possible compounds. In the top of this connector is the thermometer, which is. Use Of Distillation Thermometer.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective Use Of Distillation Thermometer In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just as it condenses. Distillation is an extremely useful technique that is used to purify reagents and separate crude product mixtures. You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about. Use Of Distillation Thermometer.
From mavink.com
Labelled Diagram Of Distillation Use Of Distillation Thermometer Distillation setup is shown in figure 1. There are two varieties of. To purify a mixture of two liquids using distillation. In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just as it condenses. Distillation is an extremely useful technique that is used to purify reagents and separate crude product. Use Of Distillation Thermometer.
From www.pinterest.com
6Plate distillation column with sight glass, thermometer, and CIP at Use Of Distillation Thermometer A distillation flask with a thermometer is placed in a heating mantle and is connected to a condenser. Distillation setup is shown in figure 1. To purify a mixture of two liquids using distillation. Distillation is an extremely useful technique that is used to purify reagents and separate crude product mixtures. In the top of this connector is the thermometer,. Use Of Distillation Thermometer.
From chem.libretexts.org
5.5D StepbyStep Procedures for Steam Distillation Chemistry LibreTexts Use Of Distillation Thermometer Distillation setup is shown in figure 1. In the top of this connector is the thermometer, which is used to read the temperature of the vapor, just as it condenses. There are two varieties of. You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see possible compounds.. Use Of Distillation Thermometer.
From copperstills.com
Thermometer Copperstills Use Of Distillation Thermometer You will receive a vial containing 11.20 g of a mixture of two compounds whose boiling points differ by about 40 °c (see possible compounds. Apply the heat source to the distilling flask. To purify a mixture of two liquids using distillation. There are two varieties of. Turn on the condenser water. A distillation flask with a thermometer is placed. Use Of Distillation Thermometer.
From chemnotcheem.com
Distillation An ancient technique to separate liquid O Level Chemistry Use Of Distillation Thermometer A distillation flask with a thermometer is placed in a heating mantle and is connected to a condenser. The tubes on the condenser are attached to a water source,. Distillation setup is shown in figure 1. Turn on the condenser water. Apply the heat source to the distilling flask. There are two varieties of. When different compounds in a. Record. Use Of Distillation Thermometer.
From www.fishersci.no
Lenz Glass Distillation Thermometer Temp. Range Use Of Distillation Thermometer Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop per second. There are two varieties of. Distillation is an extremely useful technique that is used to purify reagents and separate crude product mixtures. In the top of this connector is. Use Of Distillation Thermometer.