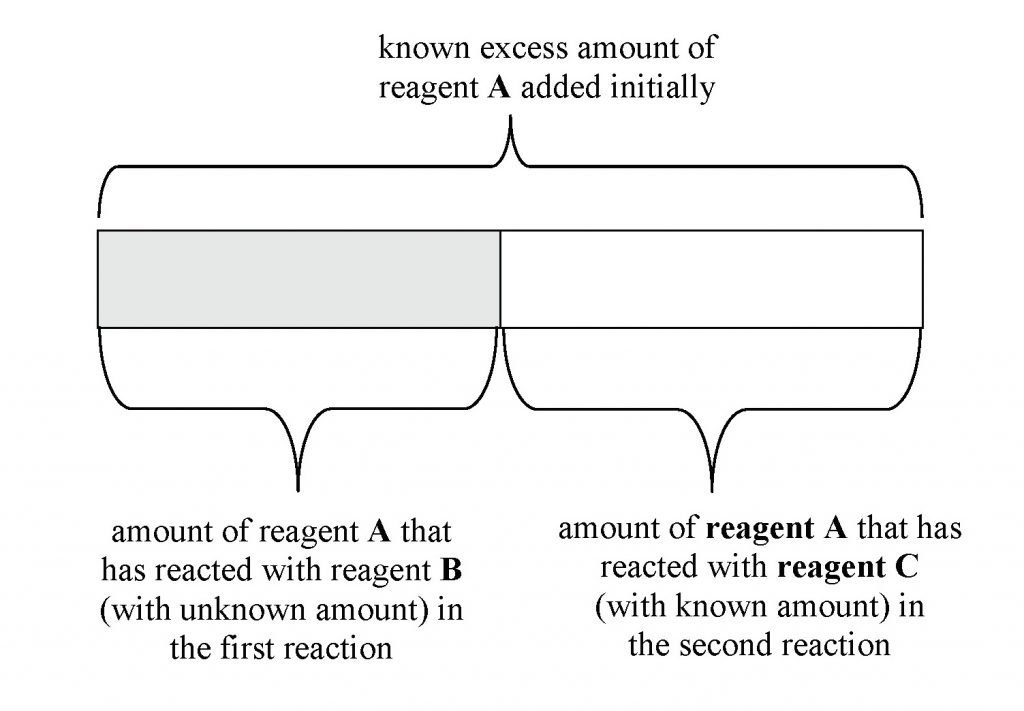
Titration Blank Definition . A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The only difference from the regular titration is the absence of. What is the equivalence point of a titration. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s volume. The main difference between back titration and direct titration is that a back titration determines the concentration of the unknown. What are titrant and analyte. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. As we have done with other titrations, we first. Learn the titration graph and titration equation.
from vasadylanpeake.blogspot.com
What are titrant and analyte. What is the equivalence point of a titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The main difference between back titration and direct titration is that a back titration determines the concentration of the unknown. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. Learn the titration graph and titration equation. As we have done with other titrations, we first. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s volume. The only difference from the regular titration is the absence of.
Back Titration Method Dylan Peake
Titration Blank Definition The only difference from the regular titration is the absence of. The only difference from the regular titration is the absence of. Learn the titration graph and titration equation. The main difference between back titration and direct titration is that a back titration determines the concentration of the unknown. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s volume. What are titrant and analyte. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. As we have done with other titrations, we first. What is the equivalence point of a titration.
From ar.inspiredpencil.com
Titration Diagram Titration Blank Definition What are titrant and analyte. Learn the titration graph and titration equation. As we have done with other titrations, we first. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. The main difference between back titration and direct titration is that a back titration determines the concentration of. Titration Blank Definition.
From labthink.netlify.app
What is titration and how does it work Titration Blank Definition As we have done with other titrations, we first. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until. Titration Blank Definition.
From chem.libretexts.org
Quantitative Analysis Nuts and Bolts Chemistry LibreTexts Titration Blank Definition A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s volume. The only difference from the regular titration is the absence of. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. As we have done with other. Titration Blank Definition.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Blank Definition As we have done with other titrations, we first. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Titration Blank Definition.
From scienceinfo.com
Titration Definition, 4 Types, Procedure Titration Blank Definition What are titrant and analyte. What is the equivalence point of a titration. Learn the titration graph and titration equation. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s volume. As we have done with other titrations, we first. The main difference between back titration and direct titration. Titration Blank Definition.
From chem-textbook.ucalgary.ca
Finding the Endpoint of a Titration UCalgary Chemistry Textbook Titration Blank Definition As we have done with other titrations, we first. What is the equivalence point of a titration. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s volume. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte.. Titration Blank Definition.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Blank Definition A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s volume. As we have done with other titrations, we first. What is the equivalence point of a titration.. Titration Blank Definition.
From www.youtube.com
Blank titration YouTube Titration Blank Definition The main difference between back titration and direct titration is that a back titration determines the concentration of the unknown. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as. Titration Blank Definition.
From www.etsy.com
Titration Flowsheet, Medication Titration Sheet, ICU Nurse, RN, BSN Etsy Titration Blank Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. What are titrant and. Titration Blank Definition.
From www.osmosis.org
Introduction to titrations Vídeo & Anatomía Osmosis Titration Blank Definition The main difference between back titration and direct titration is that a back titration determines the concentration of the unknown. What is the equivalence point of a titration. What are titrant and analyte. The only difference from the regular titration is the absence of. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as. Titration Blank Definition.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Blank Definition What are titrant and analyte. The only difference from the regular titration is the absence of. Learn the titration graph and titration equation. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. A titration is a volumetric technique in which a solution of one reactant (the titrant) is. Titration Blank Definition.
From www.chegg.com
Solved The titration hero Fill in the blanks by selecting Titration Blank Definition Learn the titration graph and titration equation. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Titration Blank Definition.
From www.slideserve.com
PPT Precipitation Titrations PowerPoint Presentation, free download Titration Blank Definition As we have done with other titrations, we first. The only difference from the regular titration is the absence of. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s volume. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Titration Blank Definition.
From www.vrogue.co
Solution Chemistry Acid Base Titration Studypool vrogue.co Titration Blank Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A blank titration is. Titration Blank Definition.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Blank Definition What is the equivalence point of a titration. The only difference from the regular titration is the absence of. The main difference between back titration and direct titration is that a back titration determines the concentration of the unknown. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s. Titration Blank Definition.
From es.slideshare.net
Tang 07 titrations 2 Titration Blank Definition As we have done with other titrations, we first. What is the equivalence point of a titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. What are titrant and analyte. The main difference between back titration and direct titration is that a back titration. Titration Blank Definition.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Titration Blank Definition A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A precipitation titration curve follows the change. Titration Blank Definition.
From vasadylanpeake.blogspot.com
Back Titration Method Dylan Peake Titration Blank Definition What are titrant and analyte. The only difference from the regular titration is the absence of. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. As we have done with other titrations, we first. What is the equivalence point of a titration. Learn the titration graph and titration. Titration Blank Definition.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Titration Blank Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. As we have done with other titrations, we first. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with. Titration Blank Definition.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Blank Definition A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. What is the equivalence point of a titration. As we have done with other titrations, we first. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Titration Blank Definition.
From www.youtube.com
blank titration in analytical chemistry YouTube Titration Blank Definition A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s volume. What is the equivalence point of a titration. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. Learn the titration graph and titration equation. A titration. Titration Blank Definition.
From hxesyopwi.blob.core.windows.net
Titration Definition In Education at Alexandra Bradford blog Titration Blank Definition What are titrant and analyte. The only difference from the regular titration is the absence of. As we have done with other titrations, we first. What is the equivalence point of a titration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until. Titration Blank Definition.
From www.youtube.com
Titration Definition, steps and formula YouTube Titration Blank Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. What are titrant and analyte. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s volume. The. Titration Blank Definition.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Blank Definition As we have done with other titrations, we first. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. The only difference from the regular titration is the absence of. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of. Titration Blank Definition.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Blank Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The main difference between back titration and direct titration is that a back titration determines the concentration of the unknown. The only difference from the regular titration is. Titration Blank Definition.
From www.numerade.com
SOLVED A blank titration is the procedure done to ensure purity and Titration Blank Definition What are titrant and analyte. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s volume. Learn the titration graph and titration equation. As we have done with other titrations, we first. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added. Titration Blank Definition.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Titration Blank Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. As we have done with other titrations, we first. What is the equivalence point of a titration. Titration is the slow addition of one solution of a known. Titration Blank Definition.
From www.numerade.com
SOLVED Define the following terms used in volumetric/titrimetric Titration Blank Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Learn the titration graph and titration equation. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s volume. A blank titration is carried out by. Titration Blank Definition.
From www.chegg.com
Solved Calculate the blankcorrected titration volume for Titration Blank Definition Learn the titration graph and titration equation. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The main difference between back titration and direct titration is that a back titration determines the concentration of the unknown. The. Titration Blank Definition.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and Titration Blank Definition Learn the titration graph and titration equation. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The only difference from the regular titration is the absence of. A precipitation titration curve follows the change in either the. Titration Blank Definition.
From hxelcilfq.blob.core.windows.net
What Is Titration In Chemistry Easy Definition at Kayla Walters blog Titration Blank Definition A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. Learn the titration graph and titration equation. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A precipitation titration curve follows the change in. Titration Blank Definition.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog Titration Blank Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The only difference from the regular titration is the absence of. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. What is the equivalence. Titration Blank Definition.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Blank Definition What is the equivalence point of a titration. A precipitation titration curve follows the change in either the titrand’s or the titrant’s concentration as a function of the titrant’s volume. What are titrant and analyte. The only difference from the regular titration is the absence of. Titration is the slow addition of one solution of a known concentration (called a. Titration Blank Definition.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Titration Blank Definition What are titrant and analyte. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Titration is. Titration Blank Definition.
From www.youtube.com
Back Titration Example YouTube Titration Blank Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. The main difference between back titration and direct titration is that a back titration determines. Titration Blank Definition.