Why Is Graphite An Element . Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed with sediment. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Since it resembles the metal lead, it is also known colloquially as. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper. Graphite has a wide range of. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. As organic material is metamorphosed, hydrogen and. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite.
from www.savemyexams.com
Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Since it resembles the metal lead, it is also known colloquially as. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper. Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed with sediment. Graphite has a wide range of. As organic material is metamorphosed, hydrogen and. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about.
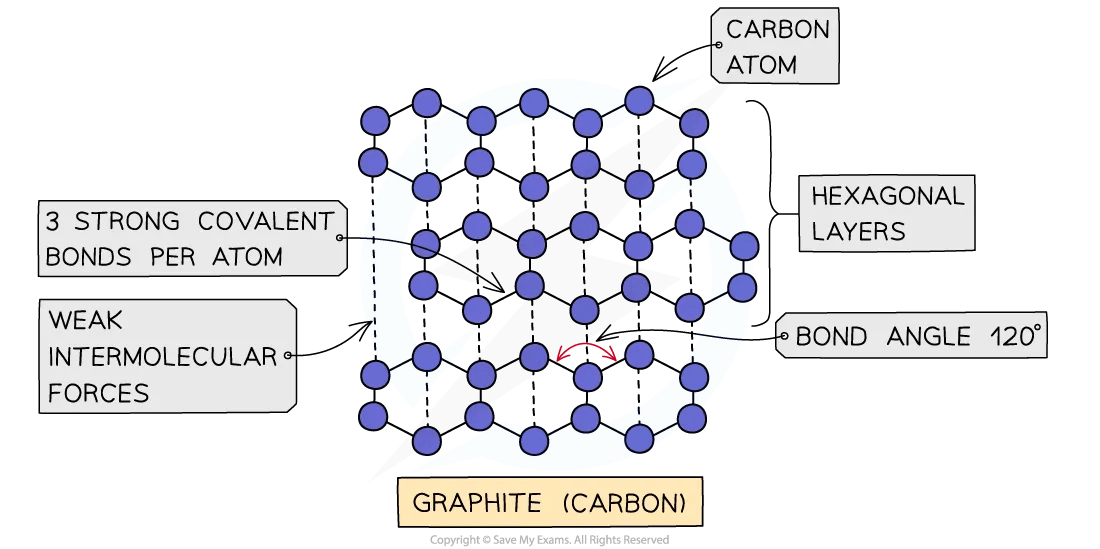
Giant Covalent Structures SL IB Chemistry Revision Notes 2025 Save
Why Is Graphite An Element Since it resembles the metal lead, it is also known colloquially as. Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed with sediment. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Since it resembles the metal lead, it is also known colloquially as. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. As organic material is metamorphosed, hydrogen and. Graphite has a wide range of.
From kamneteka.com
Камень графит его свойства, лечебное значение и влияние на разные Why Is Graphite An Element It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. As organic material is metamorphosed, hydrogen and. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Graphite has a wide range of. Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed. Why Is Graphite An Element.
From periodictable.com
Native graphite, a sample of the element Carbon in the Periodic Table Why Is Graphite An Element Graphite has a wide range of. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Since it resembles the metal lead, it is also known colloquially as. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Graphite is a mineral that. Why Is Graphite An Element.
From qrius.si.edu
Native Element Graphite Q?rius Why Is Graphite An Element Since it resembles the metal lead, it is also known colloquially as. Graphite has a wide range of. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. The. Why Is Graphite An Element.
From www.savemyexams.com
Giant Covalent Structures SL IB Chemistry Revision Notes 2025 Save Why Is Graphite An Element Graphite has a wide range of. Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed with sediment. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Since it resembles the metal lead, it is also known colloquially as. Graphite is a mineral that forms when carbon is subjected to. Why Is Graphite An Element.
From www.britannica.com
Native element Definition & Examples Britannica Why Is Graphite An Element Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Since it resembles the metal lead, it is also known colloquially as. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper. The metamorphism of carbonaceous. Why Is Graphite An Element.
From www.livescience.com
Carbon Facts about an element that is a key ingredient for life on Why Is Graphite An Element As organic material is metamorphosed, hydrogen and. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Since it resembles the metal lead, it is also known colloquially as. It can be prepared artificially by. Why Is Graphite An Element.
From www.researchgate.net
Structure of graphite. Download Scientific Diagram Why Is Graphite An Element As organic material is metamorphosed, hydrogen and. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Graphite has a wide range of. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper. Graphite is very soft because the individual layers of carbon. Why Is Graphite An Element.
From energyeducation.ca
Graphite Energy Education Why Is Graphite An Element Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Graphite has a wide range of. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. Since it resembles the metal lead, it is also known colloquially as. As organic material is metamorphosed, hydrogen and. Graphite is produced. Why Is Graphite An Element.
From gioddynci.blob.core.windows.net
Is Graphite An Element Or Compound at Charles Ferri blog Why Is Graphite An Element Graphite has a wide range of. As organic material is metamorphosed, hydrogen and. Since it resembles the metal lead, it is also known colloquially as. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Graphite is a mineral that forms when carbon is subjected to heat. Why Is Graphite An Element.
From img.hospital
Rocks & Geodes Home & Living Graphite element of the future img.hospital Why Is Graphite An Element Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed with sediment. Since it resembles the metal lead, it is also known. Why Is Graphite An Element.
From www.alamy.com
Graphite is a native element mineral composed by carbon. Sample Stock Why Is Graphite An Element Since it resembles the metal lead, it is also known colloquially as. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Graphite has a wide range of. The metamorphism of carbonaceous sediments. Why Is Graphite An Element.
From gioddynci.blob.core.windows.net
Is Graphite An Element Or Compound at Charles Ferri blog Why Is Graphite An Element As organic material is metamorphosed, hydrogen and. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Graphite has a wide range of. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce. Why Is Graphite An Element.
From www.flickr.com
Minerals Elements (Graphite) Flickr Why Is Graphite An Element Graphite has a wide range of. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the. Why Is Graphite An Element.
From energy.virginia.gov
Virginia Energy Geology and Mineral Resources Graphite Why Is Graphite An Element As organic material is metamorphosed, hydrogen and. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed with sediment. Graphite has a wide range of. Since it resembles the metal lead, it is also known colloquially as. Graphite is. Why Is Graphite An Element.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Why Is Graphite An Element As organic material is metamorphosed, hydrogen and. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. Graphite has a wide range of. Graphite is very soft because the individual layers of carbon atoms are not. Why Is Graphite An Element.
From tirupatigraphite.com
Flake Graphite Experts From India Why Is Graphite An Element Graphite has a wide range of. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. As organic material is metamorphosed, hydrogen and. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. Graphite is very soft because the individual layers of carbon atoms are not. Why Is Graphite An Element.
From www.slideserve.com
PPT Covalent Bonds PowerPoint Presentation, free download ID2654690 Why Is Graphite An Element Since it resembles the metal lead, it is also known colloquially as. As organic material is metamorphosed, hydrogen and. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Graphite is produced by metamorphosing organic material originally deposited as sediment. Why Is Graphite An Element.
From joitwiorn.blob.core.windows.net
Graphite Definition Kid Version at Nathaniel Pemberton blog Why Is Graphite An Element Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Graphite is. Why Is Graphite An Element.
From periodictable.com
Graphite from Jensan Set, a sample of the element Carbon in the Why Is Graphite An Element Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. As organic material is metamorphosed, hydrogen and. Graphite is very soft because the individual layers of carbon atoms are not as. Why Is Graphite An Element.
From www.nature-microscope-photo-video.com
Graphite Graphite Native elements Minerals Photos Why Is Graphite An Element Graphite has a wide range of. As organic material is metamorphosed, hydrogen and. Since it resembles the metal lead, it is also known colloquially as. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the. Why Is Graphite An Element.
From socratic.org
What is the chemical formula of graphite? Socratic Why Is Graphite An Element Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed with sediment. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. Graphite is the crystalline allotropic form. Why Is Graphite An Element.
From pixels.com
Graphite Molecular Structure 2 Photograph by Mikkel Juul Jensen Why Is Graphite An Element Graphite has a wide range of. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Graphite. Why Is Graphite An Element.
From mineralseducationcoalition.org
Graphite Minerals Education Coalition Why Is Graphite An Element Since it resembles the metal lead, it is also known colloquially as. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Graphite is a mineral that forms when carbon is. Why Is Graphite An Element.
From www.dreamstime.com
The structure of graphite stock illustration. Illustration of atom Why Is Graphite An Element Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. As organic material is metamorphosed, hydrogen and. Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed with sediment. Graphite has a wide range of. Graphite is a mineral that forms when carbon is. Why Is Graphite An Element.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID2754504 Why Is Graphite An Element As organic material is metamorphosed, hydrogen and. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Since it resembles the metal. Why Is Graphite An Element.
From gioddynci.blob.core.windows.net
Is Graphite An Element Or Compound at Charles Ferri blog Why Is Graphite An Element As organic material is metamorphosed, hydrogen and. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Graphite is produced by metamorphosing. Why Is Graphite An Element.
From qrius.si.edu
Native Element Graphite Q?rius Why Is Graphite An Element Graphite has a wide range of. Since it resembles the metal lead, it is also known colloquially as. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in. Why Is Graphite An Element.
From www.animalia-life.club
Graphite Crystal Structure Why Is Graphite An Element Graphite has a wide range of. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. Why Is Graphite An Element.
From superiorgraphite.com
About Graphite Graphite Products Why Is Graphite An Element The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. As organic material is metamorphosed, hydrogen and. Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed with sediment. Graphite has a wide range of. Since it resembles the metal lead, it is also known colloquially as. Graphite is a mineral. Why Is Graphite An Element.
From byjus.com
Describe the structure of Graphite. Why Is Graphite An Element Since it resembles the metal lead, it is also known colloquially as. Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed with sediment. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Graphite is the crystalline allotropic form of carbon occurs in. Why Is Graphite An Element.
From gioddynci.blob.core.windows.net
Is Graphite An Element Or Compound at Charles Ferri blog Why Is Graphite An Element Since it resembles the metal lead, it is also known colloquially as. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper. Graphite has a wide range of. Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed with sediment. Graphite is very soft because the. Why Is Graphite An Element.
From www.alamy.com
Graphite, native element Stock Photo Alamy Why Is Graphite An Element The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Since it resembles the metal lead, it is also known colloquially as. Graphite has a wide range of. As organic material is metamorphosed, hydrogen and. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. Graphite. Why Is Graphite An Element.
From www.pinterest.co.uk
graphite a form of pure carbon Rocks and Mineral Specimens for Sale Why Is Graphite An Element It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in. Why Is Graphite An Element.
From www.wikidoc.org
Allotropy wikidoc Why Is Graphite An Element As organic material is metamorphosed, hydrogen and. Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed with sediment. The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Graphite has a wide range of. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace. Why Is Graphite An Element.
From commons.wikimedia.org
FileGraphiteanddiamondwithscale.jpg Wikimedia Commons Why Is Graphite An Element Graphite is produced by metamorphosing organic material originally deposited as sediment or mixed with sediment. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust and in the upper. Since it resembles the metal lead,. Why Is Graphite An Element.