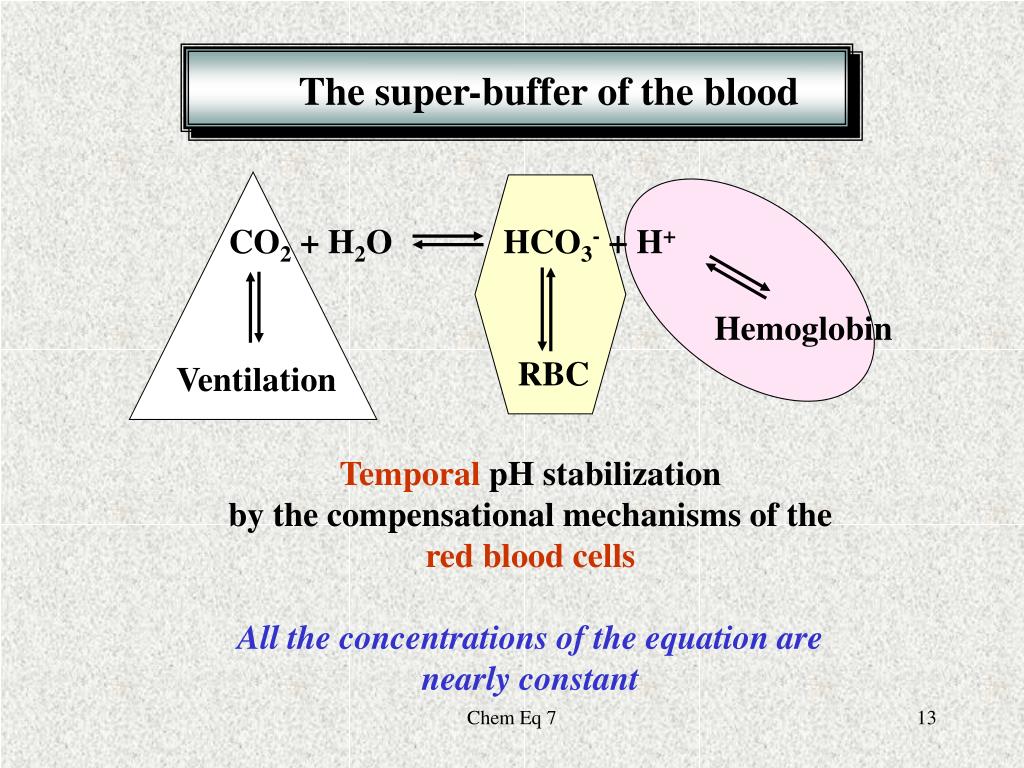
Buffer System Human Blood Is . The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Like all buffers, this system consists of weak acid/ base conjugate pairs. Human blood has a buffering system to minimize extreme changes in ph. One buffer in blood is based on the presence of hco. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. All of these contain bases that accept hydrogen. In this system, gaseous metabolic waste carbon dioxide reacts.
from www.slideserve.com
In this system, gaseous metabolic waste carbon dioxide reacts. One buffer in blood is based on the presence of hco. Human blood has a buffering system to minimize extreme changes in ph. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Like all buffers, this system consists of weak acid/ base conjugate pairs. All of these contain bases that accept hydrogen. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.
PPT Buffer Systems of the Body PowerPoint Presentation, free download
Buffer System Human Blood Is Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Human blood has a buffering system to minimize extreme changes in ph. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. All of these contain bases that accept hydrogen. Like all buffers, this system consists of weak acid/ base conjugate pairs. One buffer in blood is based on the presence of hco.
From www.slideshare.net
Blood buffer system PPT Buffer System Human Blood Is Human blood has a buffering system to minimize extreme changes in ph. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma. Buffer System Human Blood Is.
From www.slideshare.net
Buffer system Buffer System Human Blood Is In this system, gaseous metabolic waste carbon dioxide reacts. One buffer in blood is based on the presence of hco. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to. Buffer System Human Blood Is.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Buffer System Human Blood Is Like all buffers, this system consists of weak acid/ base conjugate pairs. Human blood has a buffering system to minimize extreme changes in ph. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. All of these contain bases. Buffer System Human Blood Is.
From www.slideserve.com
PPT Blood buffering system PowerPoint Presentation, free download Buffer System Human Blood Is One buffer in blood is based on the presence of hco. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. The buffer systems functioning in blood plasma include plasma proteins, phosphate,. Buffer System Human Blood Is.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 Buffer System Human Blood Is One buffer in blood is based on the presence of hco. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Like all buffers, this system consists of weak acid/ base conjugate pairs. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Human blood contains a buffer of. Buffer System Human Blood Is.
From bryont.net
Human Red Blood Cell Lysis Buffer Recipe Bryont Blog Buffer System Human Blood Is One buffer in blood is based on the presence of hco. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco. Buffer System Human Blood Is.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Buffer System Human Blood Is Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. One buffer in blood is based on the presence of hco. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon dioxide reacts. All of these contain bases that accept hydrogen. The. Buffer System Human Blood Is.
From www.paediatricsandchildhealthjournal.co.uk
Understanding blood gases/acidbase balance Paediatrics and Child Health Buffer System Human Blood Is Human blood has a buffering system to minimize extreme changes in ph. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The buffer systems functioning in blood plasma include. Buffer System Human Blood Is.
From ppt-online.org
Disorders of metabolism. (Subject 9) презентация онлайн Buffer System Human Blood Is Human blood has a buffering system to minimize extreme changes in ph. One buffer in blood is based on the presence of hco. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. All of these contain bases that accept hydrogen. The buffer systems functioning in. Buffer System Human Blood Is.
From www.slideshare.net
02 hydrolysis. buffers__colloids Buffer System Human Blood Is One buffer in blood is based on the presence of hco. Human blood has a buffering system to minimize extreme changes in ph. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate. Buffer System Human Blood Is.
From www.slideserve.com
PPT Blood buffering system PowerPoint Presentation, free download Buffer System Human Blood Is One buffer in blood is based on the presence of hco. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco. Buffer System Human Blood Is.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download Buffer System Human Blood Is In this system, gaseous metabolic waste carbon dioxide reacts. Like all buffers, this system consists of weak acid/ base conjugate pairs. One buffer in blood is based on the presence of hco. All of these contain bases that accept hydrogen. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −). Buffer System Human Blood Is.
From www.slideserve.com
PPT General, Organic, and Biochemistry, 7e PowerPoint Presentation Buffer System Human Blood Is One buffer in blood is based on the presence of hco. Human blood has a buffering system to minimize extreme changes in ph. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. All of these contain bases that accept hydrogen. Human blood contains. Buffer System Human Blood Is.
From beta.learner.org
The Buffer System in the Blood (animation) Annenberg Learner Buffer System Human Blood Is Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. All of these contain bases that accept hydrogen. Like all buffers, this system consists of weak acid/ base conjugate. Buffer System Human Blood Is.
From www.pinterest.com
Physiology Blood Buffer System Behrouz Human body facts Buffer System Human Blood Is Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. All of these contain bases that accept hydrogen. One buffer in blood is based on the presence of hco. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. In this system,. Buffer System Human Blood Is.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Buffer System Human Blood Is All of these contain bases that accept hydrogen. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in. Buffer System Human Blood Is.
From www.slideserve.com
PPT RESPIRATORY SYSTEM 3 PowerPoint Presentation, free download Buffer System Human Blood Is Human blood has a buffering system to minimize extreme changes in ph. One buffer in blood is based on the presence of hco. In this system, gaseous metabolic waste carbon dioxide reacts. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Like all buffers, this system consists of weak acid/ base conjugate pairs. Human blood. Buffer System Human Blood Is.
From www.youtube.com
Buffer in the Human Blood? What is Buffer in Chemistry? YouTube Buffer System Human Blood Is Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. All of these contain bases that accept hydrogen. In this system, gaseous metabolic waste carbon dioxide reacts. Like all buffers, this system consists of weak acid/ base conjugate pairs.. Buffer System Human Blood Is.
From www.youtube.com
Buffer action in the blood YouTube Buffer System Human Blood Is Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Like all buffers, this system. Buffer System Human Blood Is.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Buffer System Human Blood Is In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. All of these contain bases that accept hydrogen. Like all buffers, this system consists of weak acid/ base conjugate pairs. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin.. Buffer System Human Blood Is.
From resources.wfsahq.org
Respiratory Physiology Part 2 WFSA Resources Buffer System Human Blood Is All of these contain bases that accept hydrogen. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood has a buffering system to minimize extreme changes in ph. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Buffer System Human Blood Is.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory Buffer System Human Blood Is The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Like all buffers, this system consists of weak acid/ base conjugate pairs. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer. Buffer System Human Blood Is.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching Buffer System Human Blood Is Human blood has a buffering system to minimize extreme changes in ph. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. All of these contain bases that accept. Buffer System Human Blood Is.
From en.ppt-online.org
Blood biochemistry online presentation Buffer System Human Blood Is The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon dioxide reacts. All of these contain bases that accept hydrogen. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Human blood. Buffer System Human Blood Is.
From www.slideserve.com
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242 Buffer System Human Blood Is All of these contain bases that accept hydrogen. Human blood has a buffering system to minimize extreme changes in ph. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid. Buffer System Human Blood Is.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Buffer System Human Blood Is Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Human blood has a buffering system to minimize extreme changes in ph. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Like all buffers, this system consists of weak acid/ base. Buffer System Human Blood Is.
From www.chegg.com
Solved Human blood contains several buffering systems, the Buffer System Human Blood Is One buffer in blood is based on the presence of hco. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. All of these contain bases that accept hydrogen. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon. Buffer System Human Blood Is.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Buffer System Human Blood Is Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. The buffer systems functioning in. Buffer System Human Blood Is.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Buffer System Human Blood Is Human blood has a buffering system to minimize extreme changes in ph. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. All of these contain bases that accept hydrogen. The buffer systems functioning in blood plasma. Buffer System Human Blood Is.
From www.slideserve.com
PPT Biological buffering of blood PowerPoint Presentation, free Buffer System Human Blood Is The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood has a buffering system to minimize extreme changes. Buffer System Human Blood Is.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood Buffer System Human Blood Is Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. One buffer in blood is based on the presence of hco. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The buffer systems functioning in blood plasma include plasma proteins, phosphate,. Buffer System Human Blood Is.
From facts.net
20 Fascinating Facts About Blood Buffer Buffer System Human Blood Is The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Like all buffers, this system consists of weak acid/ base conjugate pairs. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. One buffer in blood is based on. Buffer System Human Blood Is.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download Buffer System Human Blood Is One buffer in blood is based on the presence of hco. Human blood has a buffering system to minimize extreme changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −). Buffer System Human Blood Is.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Buffer System Human Blood Is The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Like all buffers, this system consists of weak acid/ base conjugate pairs. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3. Buffer System Human Blood Is.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download Buffer System Human Blood Is In this system, gaseous metabolic waste carbon dioxide reacts. Like all buffers, this system consists of weak acid/ base conjugate pairs. One buffer in blood is based on the presence of hco. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Human blood has a. Buffer System Human Blood Is.