Titration Strong Acid Weak Base . In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the curve (the buffering. Titration curves for strong acid v weak base. This time we are going to use hydrochloric acid as the strong acid and ammonia solution as the weak. The reaction of the weak acid, acetic acid, with a. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Today, we will focus on the titration of a weak acid with strong bases, so we. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely dissociate in water,.
from
This time we are going to use hydrochloric acid as the strong acid and ammonia solution as the weak. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Titration curves for strong acid v weak base. The reaction of the weak acid, acetic acid, with a. Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely dissociate in water,. In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the curve (the buffering. In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). Today, we will focus on the titration of a weak acid with strong bases, so we. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh.
Titration Strong Acid Weak Base This time we are going to use hydrochloric acid as the strong acid and ammonia solution as the weak. The reaction of the weak acid, acetic acid, with a. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely dissociate in water,. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Today, we will focus on the titration of a weak acid with strong bases, so we. This time we are going to use hydrochloric acid as the strong acid and ammonia solution as the weak. In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the curve (the buffering. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). Titration curves for strong acid v weak base.
From schoolbag.info
Titration and Buffers Acids and Bases Training MCAT General Titration Strong Acid Weak Base This time we are going to use hydrochloric acid as the strong acid and ammonia solution as the weak. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially,. Titration Strong Acid Weak Base.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Strong Acid Weak Base In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). Titration curves for strong acid v weak base. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. In a titration of a weak acid/ base with a strong. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. Titration curves for strong acid v weak base. Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely dissociate in. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base The reaction of the weak acid, acetic acid, with a. In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The first example involves a strong. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base This time we are going to use hydrochloric acid as the strong acid and ammonia solution as the weak. Today, we will focus on the titration of a weak acid with strong bases, so we. In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the curve. Titration Strong Acid Weak Base.
From www.numerade.com
SOLVED The titration curve shown below is for a titration between a Titration Strong Acid Weak Base In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. The reaction of the weak acid, acetic acid, with a. This time we are going to use hydrochloric acid as the strong acid and ammonia solution as the weak. The first example involves a strong acid titration that requires. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the curve (the buffering. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. This time we are going to use hydrochloric acid as the strong. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely dissociate in water,. The reaction of the weak acid, acetic acid,. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. Titration of a strong acid with a strong base is the simplest of the four. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely dissociate in water,. Titration curves for strong acid v weak base. This time we are going to use hydrochloric acid as the strong acid and ammonia solution as the weak. The. Titration Strong Acid Weak Base.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Titration Strong Acid Weak Base The reaction of the weak acid, acetic acid, with a. Today, we will focus on the titration of a weak acid with strong bases, so we. In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the curve (the buffering. The first example involves a strong acid. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the curve (the buffering. Today, we will focus on the titration of a weak acid with strong bases, so we. The reaction of the weak acid, acetic acid, with a. This time we are going to use. Titration Strong Acid Weak Base.
From studylib.net
Titration Curve weak base with strong acid START Titration Strong Acid Weak Base The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. Titration curves for strong acid v weak base. In this particular case, the weak base. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base Today, we will focus on the titration of a weak acid with strong bases, so we. This time we are going to use hydrochloric acid as the strong acid and ammonia solution as the weak. Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and. Titration Strong Acid Weak Base.
From www.chegg.com
Solved On The Weak Acid/strong Base Titration Curve Below... Titration Strong Acid Weak Base Titration curves for strong acid v weak base. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the curve (the buffering. Titration of a strong. Titration Strong Acid Weak Base.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Strong Acid Weak Base In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). This time we are going to use hydrochloric acid as the strong acid and ammonia solution as the weak. Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base The reaction of the weak acid, acetic acid, with a. Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely dissociate in water,. Today, we will focus on the titration of a weak acid with strong bases, so we. In this. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base Today, we will focus on the titration of a weak acid with strong bases, so we. Titration curves for strong acid v weak base. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. The titration of a weak acid with a strong base involves the direct transfer of protons from the. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the curve (the buffering. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. Today, we will focus on the titration of a weak acid with. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. Today, we will focus on the titration of a weak acid with strong bases, so we. The reaction of the weak acid, acetic acid, with a. The first example involves a strong acid titration that requires only stoichiometric calculations. Titration Strong Acid Weak Base.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Strong Acid Weak Base The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. The first example involves a strong acid titration that requires only stoichiometric calculations to derive. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). This time we are going to use hydrochloric acid as the strong acid and ammonia solution as the weak. In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely dissociate in water,. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. In a titration of a weak acid/ base with a. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). Titration. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. This time we are going to use hydrochloric acid as the strong acid and ammonia solution as the weak. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the curve (the buffering. The reaction of the weak acid, acetic acid, with a. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. Titration curves for strong acid. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely dissociate in water,. This time we are going to use hydrochloric acid as. Titration Strong Acid Weak Base.
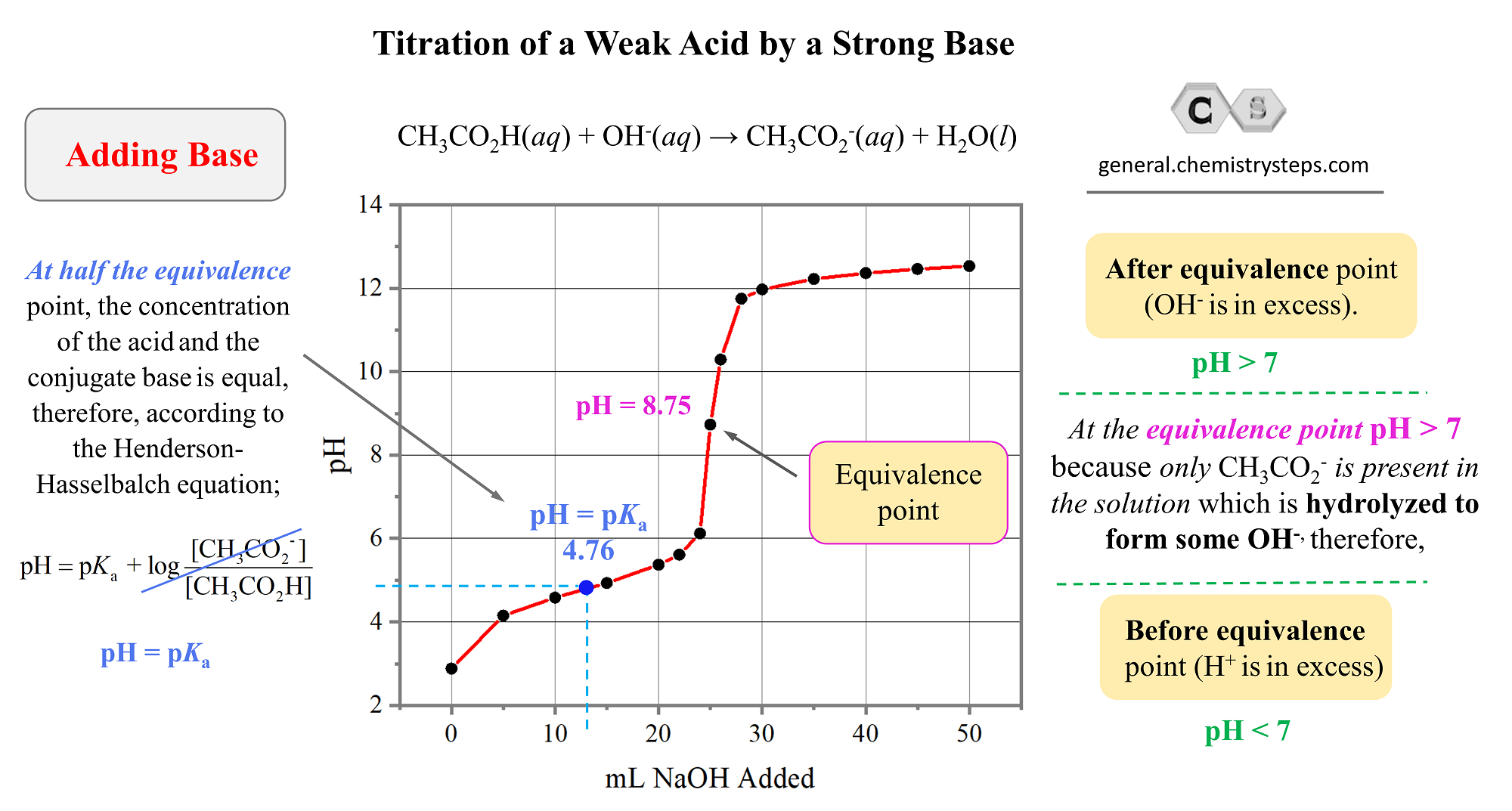
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Strong Acid Weak Base In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. Titration. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base Titration curves for strong acid v weak base. The reaction of the weak acid, acetic acid, with a. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl with 0.100 m naoh. Today, we will focus on the titration of a weak acid with strong bases, so we. This time we are going. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely dissociate in water,. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In the previous post,. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base The reaction of the weak acid, acetic acid, with a. Titration curves for strong acid v weak base. This time we are going to use hydrochloric acid as the strong acid and ammonia solution as the weak. Today, we will focus on the titration of a weak acid with strong bases, so we. Titration of a strong acid with a. Titration Strong Acid Weak Base.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titration Strong Acid Weak Base In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the curve (the buffering. Today, we will focus on the titration of a weak acid with strong bases, so we. In the previous post, we discussed the titration of 25.0 ml of 0.100 m strong acid hcl. Titration Strong Acid Weak Base.
From mungfali.com
Acid Base Titration Calculation Titration Strong Acid Weak Base Titration curves for strong acid v weak base. The reaction of the weak acid, acetic acid, with a. In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). In a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base Today, we will focus on the titration of a weak acid with strong bases, so we. Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely dissociate in water,. The first example involves a strong acid titration that requires only stoichiometric. Titration Strong Acid Weak Base.
From
Titration Strong Acid Weak Base In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely dissociate in water,. Titration curves for strong acid v weak base.. Titration Strong Acid Weak Base.