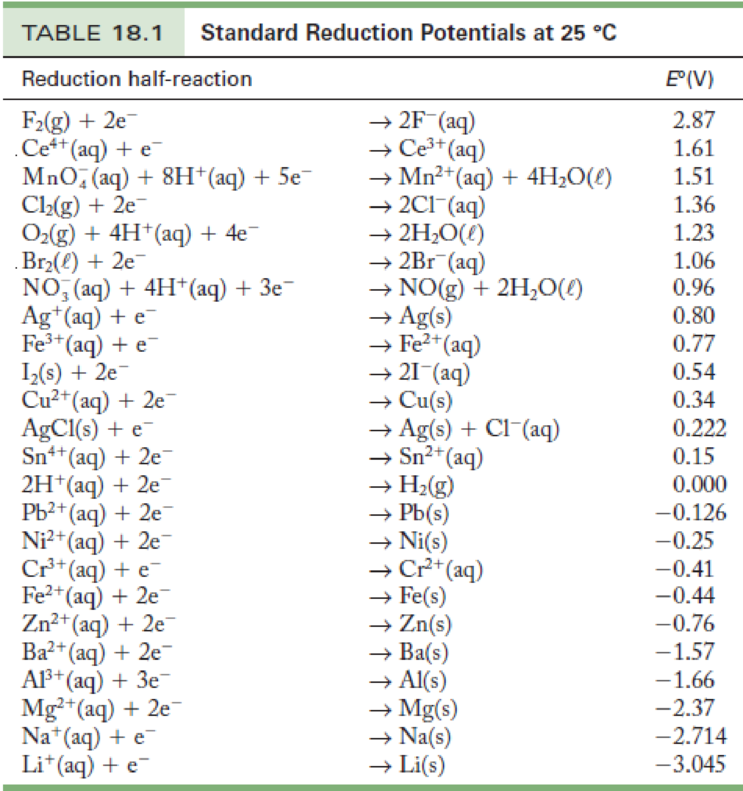
Standard Electrode Potential Data Page . You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. 45 rows standard electrode potentials in aqueous solution at 25°c. Wikipedia) t0 = 298.15 k (25.00°c) p0. The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. Standard electrode potentials in basic aqueous solution at 25 °c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Values relative to the standard hydrogen electrode (she).
from dinorahiu-images.blogspot.com
Wikipedia) t0 = 298.15 k (25.00°c) p0. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. Standard electrode potentials in basic aqueous solution at 25 °c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. 45 rows standard electrode potentials in aqueous solution at 25°c. Values relative to the standard hydrogen electrode (she).
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint
Standard Electrode Potential Data Page Wikipedia) t0 = 298.15 k (25.00°c) p0. 45 rows standard electrode potentials in aqueous solution at 25°c. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Wikipedia) t0 = 298.15 k (25.00°c) p0. Values relative to the standard hydrogen electrode (she). 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Standard electrode potentials in basic aqueous solution at 25 °c. You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or.
From www.academia.edu
(PDF) Standard Electrode Potential Aubriecia Lim Academia.edu Standard Electrode Potential Data Page The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Standard electrode potentials in basic aqueous solution at 25 °c. 45 rows standard electrode potentials in aqueous solution at 25°c.. Standard Electrode Potential Data Page.
From www.pinterest.com
Standard electrode potential (data page) Wikipedia Electrodes, Data Standard Electrode Potential Data Page Standard electrode potentials in basic aqueous solution at 25 °c. Values relative to the standard hydrogen electrode (she). Wikipedia) t0 = 298.15 k (25.00°c) p0. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 45 rows standard electrode potentials in aqueous solution at 25°c. You can change the. Standard Electrode Potential Data Page.
From pandai.me
Standard Electrode Potential Standard Electrode Potential Data Page 45 rows standard electrode potentials in aqueous solution at 25°c. Wikipedia) t0 = 298.15 k (25.00°c) p0. Values relative to the standard hydrogen electrode (she). In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. You can change the number of rows shown per page (navigate using previous and next. Standard Electrode Potential Data Page.
From www.pdffiller.com
Fillable Online The table contains some standard electrode potential Standard Electrode Potential Data Page You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. Wikipedia) t0 = 298.15 k (25.00°c) p0. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. 372 rows the data below tabulates standard electrode potentials (e. Standard Electrode Potential Data Page.
From chem.libretexts.org
1.2 Standard Electrode Potentials Chemistry LibreTexts Standard Electrode Potential Data Page 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or. Standard Electrode Potential Data Page.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Standard Electrode Potential Data Page 45 rows standard electrode potentials in aqueous solution at 25°c. You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. Wikipedia) t0 = 298.15 k (25.00°c) p0. Standard electrode potentials in basic aqueous solution at 25 °c. Values relative to the standard hydrogen electrode (she). The standard. Standard Electrode Potential Data Page.
From mungfali.com
Standard Electrode Potential Table Standard Electrode Potential Data Page The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. Standard electrode potentials in basic aqueous solution at 25 °c. Values relative to the standard hydrogen electrode (she). 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e °), in. Standard Electrode Potential Data Page.
From askfilo.com
Q.3 The graph below shows the observed standard electrode potential of so.. Standard Electrode Potential Data Page Values relative to the standard hydrogen electrode (she). 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. 45 rows standard electrode potentials in aqueous solution at 25°c. Wikipedia). Standard Electrode Potential Data Page.
From saylordotorg.github.io
Standard Potentials Standard Electrode Potential Data Page Values relative to the standard hydrogen electrode (she). Standard electrode potentials in basic aqueous solution at 25 °c. Wikipedia) t0 = 298.15 k (25.00°c) p0. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 45 rows standard electrode potentials in aqueous solution at 25°c. The standard electrode potentials. Standard Electrode Potential Data Page.
From byjus.com
6. The standard electrode potential E^° I2/I , E^° Br /Br2 and E^° Fe Standard Electrode Potential Data Page You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an. Standard Electrode Potential Data Page.
From www.chegg.com
Solved This table shows some standard electrode potential Standard Electrode Potential Data Page The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. Wikipedia) t0 = 298.15 k (25.00°c) p0. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Values relative to the standard hydrogen electrode (she). You can change the. Standard Electrode Potential Data Page.
From www.docbrown.info
electrode potential charts for transition metals 3dblock compounds Standard Electrode Potential Data Page Values relative to the standard hydrogen electrode (she). 45 rows standard electrode potentials in aqueous solution at 25°c. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Wikipedia) t0 = 298.15 k (25.00°c) p0. You can change the number of rows shown per page (navigate using previous and next. Standard Electrode Potential Data Page.
From askfilo.com
Table The Standard Electrode Potentials at 298 K Redox Reactions 57 Li+.. Standard Electrode Potential Data Page The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any. Standard Electrode Potential Data Page.
From chempedia.info
Electrode potential, standard Big Chemical Encyclopedia Standard Electrode Potential Data Page Wikipedia) t0 = 298.15 k (25.00°c) p0. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Values relative to the standard hydrogen electrode (she). Standard electrode potentials in basic aqueous solution at 25 °c. You can change the number of rows shown per page (navigate using previous and. Standard Electrode Potential Data Page.
From www.toppr.com
Using the standard electrode potentials Standard Electrode Potential Data Page You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. 45 rows standard electrode potentials in aqueous solution at 25°c. Standard electrode potentials in basic aqueous solution. Standard Electrode Potential Data Page.
From www.scribd.com
Standard Electrode Potential Series PDF Standard Electrode Potential Data Page 45 rows standard electrode potentials in aqueous solution at 25°c. Standard electrode potentials in basic aqueous solution at 25 °c. You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. Wikipedia) t0 = 298.15 k (25.00°c) p0. 372 rows the data below tabulates standard electrode potentials (e. Standard Electrode Potential Data Page.
From www.youtube.com
Using Standard Electrode Potentials YouTube Standard Electrode Potential Data Page Standard electrode potentials in basic aqueous solution at 25 °c. You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. Values relative to the standard hydrogen electrode (she). Wikipedia) t0 = 298.15 k (25.00°c) p0. In electrochemistry, standard electrode potential , or , is a measure of. Standard Electrode Potential Data Page.
From www.researchgate.net
Standard electrode potentials (E o , SHE) and the calculated values of Standard Electrode Potential Data Page 45 rows standard electrode potentials in aqueous solution at 25°c. The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. Wikipedia) t0 = 298.15 k (25.00°c) p0. You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search.. Standard Electrode Potential Data Page.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Data Page The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. In electrochemistry, standard electrode potential , or , is. Standard Electrode Potential Data Page.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Electrode Potential Data Page In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Values relative to the standard hydrogen electrode (she). Standard electrode potentials in basic aqueous solution at 25 °c. 45. Standard Electrode Potential Data Page.
From www.studypool.com
SOLUTION Standard electrode potential,definition,explanation and Standard Electrode Potential Data Page 45 rows standard electrode potentials in aqueous solution at 25°c. Values relative to the standard hydrogen electrode (she). The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. 372 rows. Standard Electrode Potential Data Page.
From www.scribd.com
Standard Electrode Potentials Standard Electrode Potential Data Page Standard electrode potentials in basic aqueous solution at 25 °c. Values relative to the standard hydrogen electrode (she). 45 rows standard electrode potentials in aqueous solution at 25°c. You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. In electrochemistry, standard electrode potential , or , is. Standard Electrode Potential Data Page.
From pt.scribd.com
Standard Electrode and Reduction Potentials at 298 K Printable Sets Standard Electrode Potential Data Page Standard electrode potentials in basic aqueous solution at 25 °c. Values relative to the standard hydrogen electrode (she). The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. 45 rows standard electrode potentials in aqueous solution at 25°c. You can change the number of rows shown per page (navigate using. Standard Electrode Potential Data Page.
From www.youtube.com
Standard electrode potential data are useful for understanding the Standard Electrode Potential Data Page Wikipedia) t0 = 298.15 k (25.00°c) p0. You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard electrode potentials are used to determine the. Standard Electrode Potential Data Page.
From www.youtube.com
APSC132 lecture 5 03 Standard Electrode Potential YouTube Standard Electrode Potential Data Page Values relative to the standard hydrogen electrode (she). In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. 45 rows standard electrode potentials in aqueous solution at 25°c. Standard electrode. Standard Electrode Potential Data Page.
From chem.libretexts.org
11.2 Standard Electrode Potentials Chemistry LibreTexts Standard Electrode Potential Data Page Standard electrode potentials in basic aqueous solution at 25 °c. 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell. Standard Electrode Potential Data Page.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Electrode Potential Data Page 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. Standard electrode potentials in basic aqueous solution at 25 °c. 45 rows standard electrode potentials in. Standard Electrode Potential Data Page.
From cbsencertsolutiononline.blogspot.com
CBSE NCERT SOLUTIONS The standard electrode potentials at 298 K Standard Electrode Potential Data Page Values relative to the standard hydrogen electrode (she). In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Wikipedia) t0 = 298.15 k (25.00°c) p0. 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to. Standard Electrode Potential Data Page.
From learnah.org
4 Measuring standard electrode potential PAG8 Standard Electrode Potential Data Page Values relative to the standard hydrogen electrode (she). 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. Wikipedia) t0 = 298.15 k (25.00°c) p0. 45 rows standard electrode. Standard Electrode Potential Data Page.
From app.pandai.org
Standard Electrode Potential Standard Electrode Potential Data Page 45 rows standard electrode potentials in aqueous solution at 25°c. Wikipedia) t0 = 298.15 k (25.00°c) p0. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. Standard electrode potentials. Standard Electrode Potential Data Page.
From mungfali.com
Table Of Standard Electrode Potentials Standard Electrode Potential Data Page Standard electrode potentials in basic aqueous solution at 25 °c. 45 rows standard electrode potentials in aqueous solution at 25°c. You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any. Standard Electrode Potential Data Page.
From www.studypool.com
SOLUTION Standard electrode potential data page Studypool Standard Electrode Potential Data Page Values relative to the standard hydrogen electrode (she). Wikipedia) t0 = 298.15 k (25.00°c) p0. The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. You can change the number of rows shown per page (navigate using previous and next at the bottom of the page), or search. In electrochemistry,. Standard Electrode Potential Data Page.
From www.alamy.com
Observed and calculated values for the standard electrode potentials Standard Electrode Potential Data Page Values relative to the standard hydrogen electrode (she). The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. Standard electrode potentials in basic aqueous solution at 25 °c. 45 rows standard electrode potentials in aqueous solution at 25°c. You can change the number of rows shown per page (navigate using. Standard Electrode Potential Data Page.
From www.studypool.com
SOLUTION Standard electrode potential data page Studypool Standard Electrode Potential Data Page The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Standard electrode potentials in basic aqueous solution at 25 °c. Wikipedia) t0 = 298.15 k (25.00°c) p0. Values relative. Standard Electrode Potential Data Page.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Data Page In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The standard electrode potentials are used to determine the electrochemical potential or the electrode potential of an electrochemical cell or. Values relative to the standard hydrogen electrode (she). You can change the number of rows shown per page (navigate using. Standard Electrode Potential Data Page.