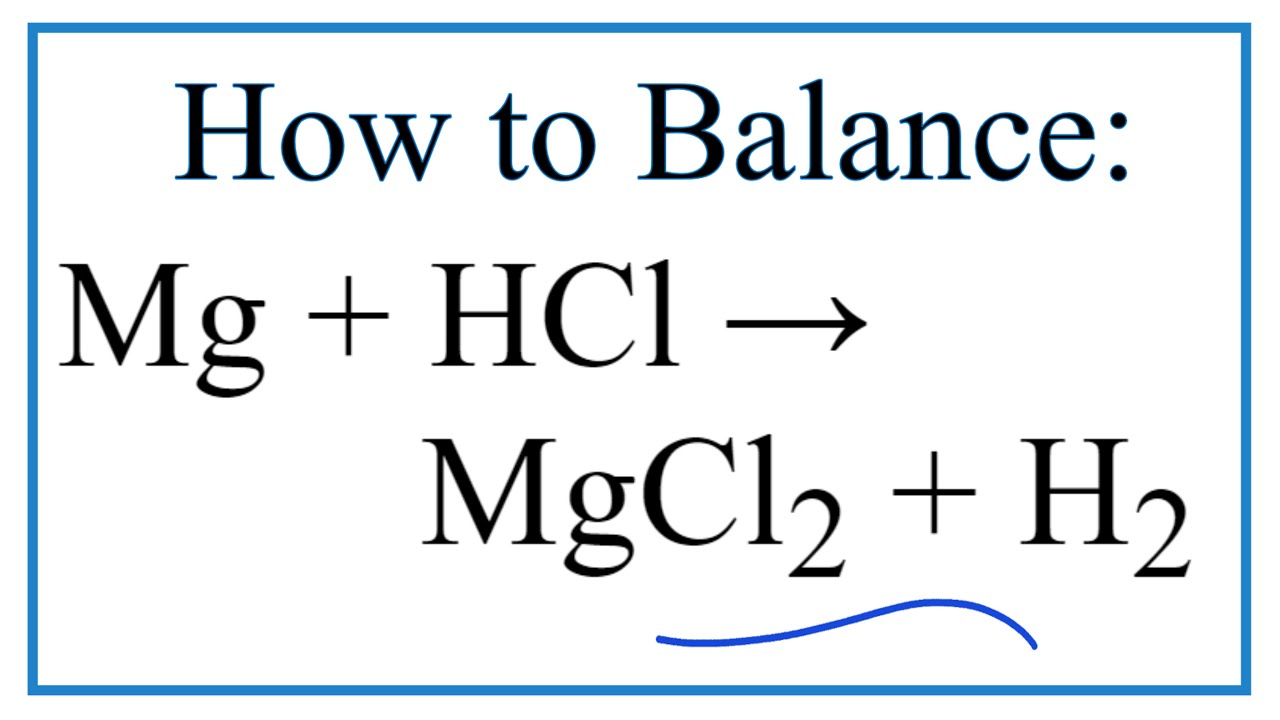
Magnesium Chloride + Hydrogen Formula . 1 mg + 2 hcl = 1 mgcl 2 + 1 h 2. Now the positive and negative charges are balanced. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. It is very important to include (aq) after the acids because the. In order to balance h on both sides we: The equation for the reaction is: Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12. Mgcl2 + h2o = mg(oh)2 + hcl is a double. Multiply coefficient for hcl by 2. We could write the chemical formula for this ionic. Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. 2 atoms in reagents and 2. Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −. Magnesium chloride + water = magnesium hydroxide + hydrogen chloride. The balanced chemical equation is:
from www.youtube.com
Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12. The equation for the reaction is: 2 atoms in reagents and 2. In order to balance h on both sides we: It is very important to include (aq) after the acids because the. We could write the chemical formula for this ionic. Now the positive and negative charges are balanced. Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −. The balanced chemical equation is:
How to Balance Mg + HCl → MgCl2 + H2 (Magnesium + Hydrochloric Acid
Magnesium Chloride + Hydrogen Formula It is very important to include (aq) after the acids because the. The balanced chemical equation is: It is very important to include (aq) after the acids because the. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. Mgcl2 + h2o = mg(oh)2 + hcl is a double. Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. In order to balance h on both sides we: Multiply coefficient for hcl by 2. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. 2 atoms in reagents and 2. 1 mg + 2 hcl = 1 mgcl 2 + 1 h 2. Magnesium chloride + water = magnesium hydroxide + hydrogen chloride. Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −. We could write the chemical formula for this ionic. Now the positive and negative charges are balanced. Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12.
From www.youtube.com
Write the chemical formula of Magnesium chloride YouTube Magnesium Chloride + Hydrogen Formula Multiply coefficient for hcl by 2. Mgcl2 + h2o = mg(oh)2 + hcl is a double. We could write the chemical formula for this ionic. Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. The balanced chemical equation is: Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −.. Magnesium Chloride + Hydrogen Formula.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium Chloride + Hydrogen Formula It is very important to include (aq) after the acids because the. Now the positive and negative charges are balanced. Multiply coefficient for hcl by 2. 1 mg + 2 hcl = 1 mgcl 2 + 1 h 2. In order to balance h on both sides we: Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g). Magnesium Chloride + Hydrogen Formula.
From www.numerade.com
SOLVEDCalcium hydrogen carbonate, magnesium chloride, and calcium Magnesium Chloride + Hydrogen Formula The equation for the reaction is: Multiply coefficient for hcl by 2. Now the positive and negative charges are balanced. It is very important to include (aq) after the acids because the. 1 mg + 2 hcl = 1 mgcl 2 + 1 h 2. We could write the chemical formula for this ionic. The balanced chemical equation is: In. Magnesium Chloride + Hydrogen Formula.
From www.numerade.com
SOLVED 1. Magnesium and hydrochloric acid react to form magnesium Magnesium Chloride + Hydrogen Formula In order to balance h on both sides we: 1 mg + 2 hcl = 1 mgcl 2 + 1 h 2. Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12. The equation for the reaction is: Multiply coefficient for hcl by. Magnesium Chloride + Hydrogen Formula.
From www.numerade.com
SOLVED When hydrochloric acid reacts with magnesium metal, hydrogen Magnesium Chloride + Hydrogen Formula Magnesium + hydrochloric acid → magnesium chloride + hydrogen. The balanced chemical equation is: Multiply coefficient for hcl by 2. 1 mg + 2 hcl = 1 mgcl 2 + 1 h 2. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. It is very important to include (aq) after the acids. Magnesium Chloride + Hydrogen Formula.
From www.slideserve.com
PPT Mg(OH) 2 + 2HCl → 2H 2 O + MgCl 2 PowerPoint Presentation, free Magnesium Chloride + Hydrogen Formula Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −. Multiply coefficient for hcl by 2. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. The equation for the reaction is: Mgcl2 + h2o = mg(oh)2 + hcl is a double. The balanced chemical equation is: Mg (s) + 2hcl (aq) →. Magnesium Chloride + Hydrogen Formula.
From askfilo.com
Examples 1. Formula of hydrogen chloride Formula of the compound would be.. Magnesium Chloride + Hydrogen Formula Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −. 2 atoms in reagents and 2. In order to balance h on both sides we: Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Now the positive and negative charges are balanced. 1 mg + 2 hcl = 1 mgcl 2 + 1 h 2. Magnesium chloride is an inorganic. Magnesium Chloride + Hydrogen Formula.
From armsingle10.pythonanywhere.com
Supreme Mgcl2 And Water Equation All Formulas Of Science Class 9 Ncert Magnesium Chloride + Hydrogen Formula Multiply coefficient for hcl by 2. It is very important to include (aq) after the acids because the. Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −. 2 atoms in reagents and 2. We could write the chemical formula for this ionic. The balanced chemical equation is: Now the positive and negative charges are balanced. Magnesium chloride + water. Magnesium Chloride + Hydrogen Formula.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Magnesium Chloride + Hydrogen Formula Magnesium chloride + water = magnesium hydroxide + hydrogen chloride. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12. 1 mg + 2 hcl = 1 mgcl 2 + 1 h 2. The. Magnesium Chloride + Hydrogen Formula.
From www.bartleby.com
Answered Magnesium metal reacts with… bartleby Magnesium Chloride + Hydrogen Formula Now the positive and negative charges are balanced. 2 atoms in reagents and 2. The equation for the reaction is: 1 mg + 2 hcl = 1 mgcl 2 + 1 h 2. Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12.. Magnesium Chloride + Hydrogen Formula.
From www.slideserve.com
PPT Balancing Chemical Reactions PowerPoint Presentation, free Magnesium Chloride + Hydrogen Formula Multiply coefficient for hcl by 2. Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −. The balanced chemical equation is: 2 atoms in reagents and 2. We could write the chemical formula for this ionic. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. Magnesium chloride + water = magnesium hydroxide. Magnesium Chloride + Hydrogen Formula.
From www.sarthaks.com
Write down the formulae of these compounds, using criss cross method Magnesium Chloride + Hydrogen Formula Multiply coefficient for hcl by 2. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. Mgcl2 + h2o = mg(oh)2 + hcl is a double. It is very important to include (aq) after the acids because the. Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −. Mg (s) + 2hcl (aq). Magnesium Chloride + Hydrogen Formula.
From www.slideserve.com
PPT NOTES 2 Binary Ionic Chemical Names and Formulas PowerPoint Magnesium Chloride + Hydrogen Formula 2 atoms in reagents and 2. The balanced chemical equation is: Multiply coefficient for hcl by 2. 1 mg + 2 hcl = 1 mgcl 2 + 1 h 2. Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12. Mg2+cl−cl− (4.3.2) (4.3.2). Magnesium Chloride + Hydrogen Formula.
From www.numerade.com
SOLVED Magnesium reacts with hydrochloric acid, HCl, to form magnesium Magnesium Chloride + Hydrogen Formula Multiply coefficient for hcl by 2. Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −. Now the positive and negative charges are balanced. Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. 1 mg + 2 hcl = 1 mgcl 2 + 1 h 2. 2 atoms in. Magnesium Chloride + Hydrogen Formula.
From www.numerade.com
SOLVED Question 28 What is a possible product in the reaction of pure Magnesium Chloride + Hydrogen Formula 2 atoms in reagents and 2. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. We could write the chemical formula for this ionic. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. Now the positive and negative charges are balanced. Magnesium chloride + water = magnesium hydroxide + hydrogen chloride. 1. Magnesium Chloride + Hydrogen Formula.
From www.numerade.com
SOLVEDZinc and magnesium metal each reacts with hydrochloric acid to Magnesium Chloride + Hydrogen Formula Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. It is very important to include (aq) after the acids because the. The equation for the reaction is: Magnesium + hydrochloric acid →. Magnesium Chloride + Hydrogen Formula.
From blog.iceslicer.com
Chloride Spotlight What is Magnesium Chloride? Magnesium Chloride + Hydrogen Formula It is very important to include (aq) after the acids because the. Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12. The balanced chemical equation is: Mgcl2 + h2o = mg(oh)2 + hcl is a double. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_. Magnesium Chloride + Hydrogen Formula.
From www.youtube.com
Write the chemical formula of the following compounds (a)Magnesium Magnesium Chloride + Hydrogen Formula Magnesium + hydrochloric acid → magnesium chloride + hydrogen. We could write the chemical formula for this ionic. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. The equation for the reaction is: Magnesium chloride + water = magnesium hydroxide + hydrogen chloride. Multiply coefficient for hcl by 2. Now the positive. Magnesium Chloride + Hydrogen Formula.
From brainly.in
Write the formation of magnesium chloride (MgCl,) with the help of Magnesium Chloride + Hydrogen Formula Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12. Now the positive and negative charges are balanced. Multiply coefficient for hcl by. Magnesium Chloride + Hydrogen Formula.
From www.numerade.com
SOLVED (a) Write the balanced chemical equation following reaction. 1 Magnesium Chloride + Hydrogen Formula 2 atoms in reagents and 2. Mgcl2 + h2o = mg(oh)2 + hcl is a double. We could write the chemical formula for this ionic. Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. Multiply coefficient for hcl by 2. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) +. Magnesium Chloride + Hydrogen Formula.
From www.numerade.com
SOLVED What will cause more Magnesium to be dissolved in a solution of Magnesium Chloride + Hydrogen Formula Mgcl2 + h2o = mg(oh)2 + hcl is a double. The balanced chemical equation is: The equation for the reaction is: 2 atoms in reagents and 2. It is very important to include (aq) after the acids because the. Multiply coefficient for hcl by 2. Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −. Magnesium chloride is an inorganic. Magnesium Chloride + Hydrogen Formula.
From www.chemicalslearning.com
Magnesium Chloride Formula, Properties and Uses Magnesium Chloride + Hydrogen Formula Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. Multiply coefficient for hcl by 2. It is very important to include (aq) after the acids because the. Mgcl2 + h2o = mg(oh)2 + hcl is a double. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Now the positive and negative charges. Magnesium Chloride + Hydrogen Formula.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Magnesium Chloride + Hydrogen Formula We could write the chemical formula for this ionic. Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12. Now the positive and negative charges are balanced. Multiply coefficient for hcl by 2. Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −.. Magnesium Chloride + Hydrogen Formula.
From mavink.com
Magnesium And Hcl Reaction Magnesium Chloride + Hydrogen Formula In order to balance h on both sides we: Multiply coefficient for hcl by 2. Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. We could write the chemical formula for this ionic. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Magnesium chloride is an inorganic compound with. Magnesium Chloride + Hydrogen Formula.
From www.numerade.com
SOLVED Magnesium metal reacts with hydrochloric acid to produce Magnesium Chloride + Hydrogen Formula Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12. Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. Multiply coefficient for hcl by 2. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2. Magnesium Chloride + Hydrogen Formula.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science hive Magnesium Chloride + Hydrogen Formula Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −. 1 mg + 2 hcl = 1 mgcl 2 + 1 h 2. It is very important to include (aq) after the acids because the. In order to balance h on both sides we: The balanced chemical equation is: Mg (s). Magnesium Chloride + Hydrogen Formula.
From www.vrogue.co
Chemical Formula Of The Following Compounds By Crissc vrogue.co Magnesium Chloride + Hydrogen Formula Multiply coefficient for hcl by 2. Now the positive and negative charges are balanced. Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. We could. Magnesium Chloride + Hydrogen Formula.
From mammothmemory.net
Similarly magnesium and hydrochloric acid is a slow reaction Magnesium Chloride + Hydrogen Formula 2 atoms in reagents and 2. Now the positive and negative charges are balanced. The balanced chemical equation is: We could write the chemical formula for this ionic. Mg2+cl−cl− (4.3.2) (4.3.2) mg 2 + cl − cl −. Multiply coefficient for hcl by 2. In order to balance h on both sides we: Mg (s) + 2hcl (aq) → mgcl. Magnesium Chloride + Hydrogen Formula.
From www.numerade.com
SOLVED Magnesium metal reacts with chlorine gas, Cl2, to produce Magnesium Chloride + Hydrogen Formula Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. 2 atoms in reagents and 2. The equation for the reaction is: Mgcl2 + h2o = mg(oh)2 + hcl is a double. It is very important to include (aq) after the acids because the. The balanced chemical equation is: Mg (s) + 2hcl. Magnesium Chloride + Hydrogen Formula.
From byjus.com
By the transfer of electrons, illustrate the formation of bond in Magnesium Chloride + Hydrogen Formula Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12. 1 mg + 2 hcl = 1 mgcl 2 + 1 h 2. Magnesium chloride + water = magnesium hydroxide + hydrogen chloride. We could write the chemical formula for this ionic. Now. Magnesium Chloride + Hydrogen Formula.
From www.slideserve.com
PPT Rate of Reactions PowerPoint Presentation, free download ID6722004 Magnesium Chloride + Hydrogen Formula Magnesium + hydrochloric acid → magnesium chloride + hydrogen. It is very important to include (aq) after the acids because the. Now the positive and negative charges are balanced. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. The balanced chemical equation is: Multiply coefficient for hcl by 2. Mg2+cl−cl− (4.3.2) (4.3.2). Magnesium Chloride + Hydrogen Formula.
From astonishingceiyrs.blogspot.com
Magnesium Chloride Formula astonishingceiyrs Magnesium Chloride + Hydrogen Formula Now the positive and negative charges are balanced. In order to balance h on both sides we: 2 atoms in reagents and 2. The balanced chemical equation is: Mgcl2 + h2o = mg(oh)2 + hcl is a double. Magnesium chloride + water = magnesium hydroxide + hydrogen chloride. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g). Magnesium Chloride + Hydrogen Formula.
From www.youtube.com
How to Balance Mg + HCl → MgCl2 + H2 (Magnesium + Hydrochloric Acid Magnesium Chloride + Hydrogen Formula Now the positive and negative charges are balanced. It is very important to include (aq) after the acids because the. Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. Mg_ ( (s))+ 2hcl_ ( (aq))rarrmgcl_ (2 (aq)) + h_ (2 (g) the reaction between magnesium and. 1 mg +. Magnesium Chloride + Hydrogen Formula.
From www.chegg.com
Solved Magnesium metal reacts with hydrochloric acid to Magnesium Chloride + Hydrogen Formula The balanced chemical equation is: It is very important to include (aq) after the acids because the. Mgcl2 + h2o = mg(oh)2 + hcl is a double. Now the positive and negative charges are balanced. Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. In order to balance h. Magnesium Chloride + Hydrogen Formula.
From www.numerade.com
SOLVED Solid magnesium reacts with hydrochloric acid (HCI) to form Magnesium Chloride + Hydrogen Formula Mgcl2 + h2o = mg(oh)2 + hcl is a double. 2 atoms in reagents and 2. In order to balance h on both sides we: Now the positive and negative charges are balanced. Magnesium chloride is an inorganic compound with the formula mg cl 2.it forms hydrates mgcl 2 ·nh 2 o, where n can range from 1 to 12.. Magnesium Chloride + Hydrogen Formula.