Magnesium Ion Formula Unit . What is the chemical formula of the magnesium ion? Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons. A chemical formula is an expression used to represent an atom, ion, or compound. Ionic compound close ionic compoundan ionic compound occurs when a negative ion (an atom that has gained an. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. This is two positive charges and two negative charges. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The atom that the ion is formed from as well as the charge of the ion is both. Predict the charge of monatomic main group elements based on their group number. The symbol for the ion is mg 2+, and it is called a magnesium ion. Write formulas for ionic compounds.
from www.vectorstock.com
When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The symbol for the ion is mg 2+, and it is called a magnesium ion. What is the chemical formula of the magnesium ion? Predict the charge of monatomic main group elements based on their group number. A chemical formula is an expression used to represent an atom, ion, or compound. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. The atom that the ion is formed from as well as the charge of the ion is both. Ionic compound close ionic compoundan ionic compound occurs when a negative ion (an atom that has gained an. This is two positive charges and two negative charges. For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons.
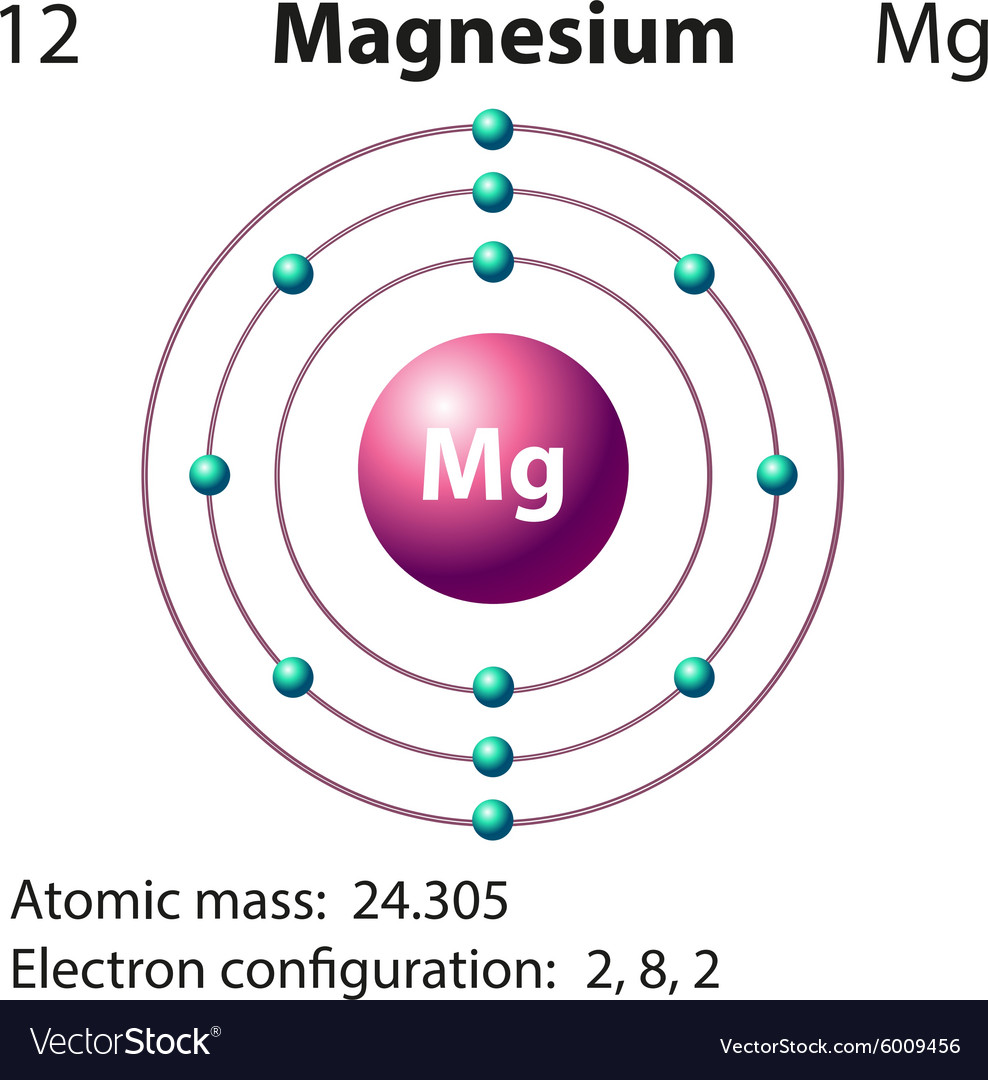
Diagram representation of the element magnesium Vector Image
Magnesium Ion Formula Unit Predict the charge of monatomic main group elements based on their group number. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Ionic compound close ionic compoundan ionic compound occurs when a negative ion (an atom that has gained an. For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons. Predict the charge of monatomic main group elements based on their group number. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. The symbol for the ion is mg 2+, and it is called a magnesium ion. This is two positive charges and two negative charges. Write formulas for ionic compounds. A chemical formula is an expression used to represent an atom, ion, or compound. What is the chemical formula of the magnesium ion? When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The atom that the ion is formed from as well as the charge of the ion is both.
From www.slideshare.net
Bonding Basics Magnesium Ion Formula Unit Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Predict the charge of monatomic main group elements based on their group number. Write formulas for ionic compounds. A chemical formula is an expression used to represent an atom, ion, or compound. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has. Magnesium Ion Formula Unit.
From www.youtube.com
How to find the Oxidation Number for the Mg2+ ion. (Magnesium ion Magnesium Ion Formula Unit The atom that the ion is formed from as well as the charge of the ion is both. Write formulas for ionic compounds. Ionic compound close ionic compoundan ionic compound occurs when a negative ion (an atom that has gained an. This is two positive charges and two negative charges. The symbol for the ion is mg 2+, and it. Magnesium Ion Formula Unit.
From www.britannica.com
magnesium Description, Properties, & Compounds Britannica Magnesium Ion Formula Unit For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons. This is two positive charges and two negative charges. The symbol for the ion is mg 2+, and it is called a magnesium ion. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Write formulas for ionic compounds. A. Magnesium Ion Formula Unit.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Ion Formula Unit When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. What is the chemical formula of the magnesium ion? When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. This is two. Magnesium Ion Formula Unit.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Magnesium Ion Formula Unit Predict the charge of monatomic main group elements based on their group number. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. The atom that the ion is formed from as. Magnesium Ion Formula Unit.
From www.youtube.com
Draw the Lewis Structure of MgI2 (magnesium iodide) YouTube Magnesium Ion Formula Unit For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons. A chemical formula is an expression used to represent an atom, ion, or compound. This is two positive charges and two negative charges. Ionic compound close ionic compoundan ionic compound occurs when a negative ion (an atom that has gained an. Nitrogen’s position in. Magnesium Ion Formula Unit.
From www.numerade.com
SOLVED Arrange the compounds in order of increasing number of oxygen Magnesium Ion Formula Unit This is two positive charges and two negative charges. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Predict the charge of monatomic main group elements based on their group number. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has. Magnesium Ion Formula Unit.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Magnesium Ion Formula Unit When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Predict the charge of monatomic main group elements based on their group number. A chemical formula is an expression used to represent an atom, ion, or compound. Write formulas for ionic compounds. What is the chemical. Magnesium Ion Formula Unit.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Ion Formula Unit When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Ionic compound close ionic compoundan ionic compound occurs when a negative ion (an atom that has gained an. The atom that the ion is formed from as well as the charge of the ion is both.. Magnesium Ion Formula Unit.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion Formula Unit Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Write formulas for ionic compounds. This is two positive charges and two negative charges. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The atom that the ion is formed from. Magnesium Ion Formula Unit.
From www.pearson.com
Show the complete orbital diagram of magnesium. Channels for Pearson+ Magnesium Ion Formula Unit Predict the charge of monatomic main group elements based on their group number. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. What is the chemical formula of the magnesium ion? For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses. Magnesium Ion Formula Unit.
From brainly.com
⚗️What is the electron configuration for the Magnesium ion? Magnesium Ion Formula Unit Predict the charge of monatomic main group elements based on their group number. This is two positive charges and two negative charges. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. What is the chemical formula of the magnesium ion? When an ionic compound is. Magnesium Ion Formula Unit.
From www.newtondesk.com
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards Magnesium Ion Formula Unit When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. A chemical formula is an expression used to represent an atom, ion, or compound. For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons. The symbol for the ion is. Magnesium Ion Formula Unit.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Magnesium Ion Formula Unit When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Write formulas for ionic compounds. This is two positive charges and two negative charges. Ionic compound close ionic compoundan ionic compound occurs when a negative ion (an atom that has gained an. A chemical formula is. Magnesium Ion Formula Unit.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Ion Formula Unit Predict the charge of monatomic main group elements based on their group number. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The symbol for the ion is mg 2+, and it is called a magnesium ion. Write formulas for ionic compounds. The atom that. Magnesium Ion Formula Unit.
From 88guru.com
What is formula unit mass. How is it Calculated? 88Guru Magnesium Ion Formula Unit Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Predict the charge of monatomic main group elements based on their group number. For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons. Write formulas for ionic compounds. The atom that the ion is formed from as well as the. Magnesium Ion Formula Unit.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Ion Formula Unit The symbol for the ion is mg 2+, and it is called a magnesium ion. The atom that the ion is formed from as well as the charge of the ion is both. What is the chemical formula of the magnesium ion? Ionic compound close ionic compoundan ionic compound occurs when a negative ion (an atom that has gained an.. Magnesium Ion Formula Unit.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Formula Unit This is two positive charges and two negative charges. Predict the charge of monatomic main group elements based on their group number. A chemical formula is an expression used to represent an atom, ion, or compound. The atom that the ion is formed from as well as the charge of the ion is both. The symbol for the ion is. Magnesium Ion Formula Unit.
From www.neuroneeds.com
MAGNESIUM Neuroneeds Magnesium Ion Formula Unit For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons. Predict the charge of monatomic main group elements based on their group number. What is the chemical formula of the magnesium ion? When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. Magnesium Ion Formula Unit.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Formula Unit For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. A chemical formula is an expression used to represent an atom, ion, or compound. The atom that the ion is. Magnesium Ion Formula Unit.
From www.slideserve.com
PPT Atom the smallest unit of matter “indivisible” PowerPoint Magnesium Ion Formula Unit When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Predict the charge of monatomic main group elements based on their group number. A chemical formula is an expression used to represent an atom, ion, or compound. Write formulas for ionic compounds. The symbol for the. Magnesium Ion Formula Unit.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Ion Formula Unit The atom that the ion is formed from as well as the charge of the ion is both. The symbol for the ion is mg 2+, and it is called a magnesium ion. For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons. When an ionic compound is formed from magnesium and oxygen, the. Magnesium Ion Formula Unit.
From brainly.in
EXPLAIN WHY MAGNESIUM FORMS MG ION Brainly.in Magnesium Ion Formula Unit What is the chemical formula of the magnesium ion? For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons. The symbol for the ion is mg 2+, and it is called a magnesium ion. Write formulas for ionic compounds. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a. Magnesium Ion Formula Unit.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Ion Formula Unit Write formulas for ionic compounds. Predict the charge of monatomic main group elements based on their group number. The atom that the ion is formed from as well as the charge of the ion is both. What is the chemical formula of the magnesium ion? When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a. Magnesium Ion Formula Unit.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Formula Unit This is two positive charges and two negative charges. Predict the charge of monatomic main group elements based on their group number. For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. Magnesium Ion Formula Unit.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Formula Unit A chemical formula is an expression used to represent an atom, ion, or compound. Write formulas for ionic compounds. This is two positive charges and two negative charges. The atom that the ion is formed from as well as the charge of the ion is both. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has. Magnesium Ion Formula Unit.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Ion Formula Unit What is the chemical formula of the magnesium ion? A chemical formula is an expression used to represent an atom, ion, or compound. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. When an ionic compound is formed from magnesium and oxygen, the magnesium ion. Magnesium Ion Formula Unit.
From www.youtube.com
Writing Ionic Formulas Magnesium Nitride YouTube Magnesium Ion Formula Unit The atom that the ion is formed from as well as the charge of the ion is both. The symbol for the ion is mg 2+, and it is called a magnesium ion. This is two positive charges and two negative charges. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and. Magnesium Ion Formula Unit.
From www.youtube.com
How to Find the Ionic Charge for Magnesium (Mg) YouTube Magnesium Ion Formula Unit When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Ionic compound close ionic compoundan ionic compound occurs when a negative ion (an atom that has gained an. This is two positive. Magnesium Ion Formula Unit.
From www.thesciencehive.co.uk
The Periodic Table (GCSE) — the science hive Magnesium Ion Formula Unit For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons. This is two positive charges and two negative charges. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Nitrogen’s position in the periodic table (group 15) reveals that it. Magnesium Ion Formula Unit.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Magnesium Ion Formula Unit The symbol for the ion is mg 2+, and it is called a magnesium ion. Write formulas for ionic compounds. Ionic compound close ionic compoundan ionic compound occurs when a negative ion (an atom that has gained an. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. The atom that the ion is formed from. Magnesium Ion Formula Unit.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 Magnesium Ion Formula Unit When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Ionic compound close ionic compoundan ionic compound occurs when a negative ion (an atom that has gained an. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Predict the charge of. Magnesium Ion Formula Unit.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion Formula Unit When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Ionic compound close ionic compoundan ionic compound occurs when a negative ion. Magnesium Ion Formula Unit.
From mataseluruhdunia202.blogspot.com
Modelo Atomico De Magnesio Animal Magnesium Ion Formula Unit What is the chemical formula of the magnesium ion? For example, a magnesium ion (mg 2+) is formed when a magnesium atom loses two electrons. Predict the charge of monatomic main group elements based on their group number. This is two positive charges and two negative charges. When an ionic compound is formed from magnesium and oxygen, the magnesium ion. Magnesium Ion Formula Unit.
From www.alamy.com
Chemical element magnesium hires stock photography and images Alamy Magnesium Ion Formula Unit This is two positive charges and two negative charges. The symbol for the ion is mg 2+, and it is called a magnesium ion. Write formulas for ionic compounds. What is the chemical formula of the magnesium ion? The atom that the ion is formed from as well as the charge of the ion is both. Ionic compound close ionic. Magnesium Ion Formula Unit.