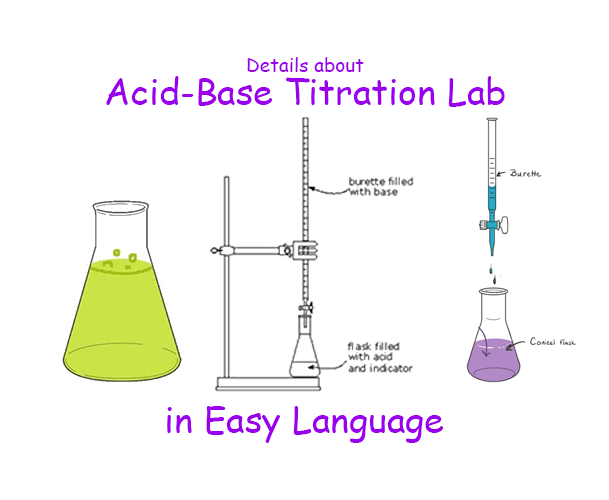
The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why . If a large amount of. A known volume of the unknown concentration solution should be placed in a beaker under the burette. The h is the proton which can be given. It is based on the neutralization reaction, where. These traits are desirable so only a small amount of an indicator is needed. To do this you should add a small. Litmus is a weak acid. A useful indicator has a strong color that changes quickly near its pka. It has a seriously complicated molecule which we will simplify to hlit.
from mungfali.com
The h is the proton which can be given. These traits are desirable so only a small amount of an indicator is needed. If a large amount of. A known volume of the unknown concentration solution should be placed in a beaker under the burette. It has a seriously complicated molecule which we will simplify to hlit. A useful indicator has a strong color that changes quickly near its pka. It is based on the neutralization reaction, where. To do this you should add a small. Litmus is a weak acid.
Acid Base Titration Calculation
The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why It is based on the neutralization reaction, where. To do this you should add a small. Litmus is a weak acid. It has a seriously complicated molecule which we will simplify to hlit. A known volume of the unknown concentration solution should be placed in a beaker under the burette. These traits are desirable so only a small amount of an indicator is needed. The h is the proton which can be given. A useful indicator has a strong color that changes quickly near its pka. It is based on the neutralization reaction, where. If a large amount of.
From mungfali.com
Acid Base Titration Indicator The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why To do this you should add a small. These traits are desirable so only a small amount of an indicator is needed. The h is the proton which can be given. A useful indicator has a strong color that changes quickly near its pka. It has a seriously complicated molecule which we will simplify to hlit. If a large amount. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why A known volume of the unknown concentration solution should be placed in a beaker under the burette. It has a seriously complicated molecule which we will simplify to hlit. It is based on the neutralization reaction, where. A useful indicator has a strong color that changes quickly near its pka. These traits are desirable so only a small amount of. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why It has a seriously complicated molecule which we will simplify to hlit. It is based on the neutralization reaction, where. The h is the proton which can be given. These traits are desirable so only a small amount of an indicator is needed. Litmus is a weak acid. A known volume of the unknown concentration solution should be placed in. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.studypool.com
SOLUTION Acids bases titration indicators and titration curves Studypool The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why A known volume of the unknown concentration solution should be placed in a beaker under the burette. To do this you should add a small. It has a seriously complicated molecule which we will simplify to hlit. The h is the proton which can be given. It is based on the neutralization reaction, where. If a large amount of. These. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why Litmus is a weak acid. To do this you should add a small. A useful indicator has a strong color that changes quickly near its pka. It is based on the neutralization reaction, where. It has a seriously complicated molecule which we will simplify to hlit. These traits are desirable so only a small amount of an indicator is needed.. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why It has a seriously complicated molecule which we will simplify to hlit. Litmus is a weak acid. A useful indicator has a strong color that changes quickly near its pka. To do this you should add a small. The h is the proton which can be given. A known volume of the unknown concentration solution should be placed in a. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why The h is the proton which can be given. A known volume of the unknown concentration solution should be placed in a beaker under the burette. Litmus is a weak acid. A useful indicator has a strong color that changes quickly near its pka. It is based on the neutralization reaction, where. It has a seriously complicated molecule which we. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.biorender.com
AcidBase Titration BioRender Science Templates The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why These traits are desirable so only a small amount of an indicator is needed. Litmus is a weak acid. It is based on the neutralization reaction, where. A known volume of the unknown concentration solution should be placed in a beaker under the burette. If a large amount of. The h is the proton which can be given. To do. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.numerade.com
SOLVED Qualitatively, describe how an acidbase indicator works. Why The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why A known volume of the unknown concentration solution should be placed in a beaker under the burette. Litmus is a weak acid. It has a seriously complicated molecule which we will simplify to hlit. If a large amount of. A useful indicator has a strong color that changes quickly near its pka. The h is the proton which can be. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From chem-textbook.ucalgary.ca
AcidBase Colour Indicators UCalgary Chemistry Textbook The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why These traits are desirable so only a small amount of an indicator is needed. To do this you should add a small. Litmus is a weak acid. If a large amount of. The h is the proton which can be given. It is based on the neutralization reaction, where. A known volume of the unknown concentration solution should be placed. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From mungfali.com
Acid Base Titration Experiment The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why A useful indicator has a strong color that changes quickly near its pka. If a large amount of. These traits are desirable so only a small amount of an indicator is needed. It has a seriously complicated molecule which we will simplify to hlit. Litmus is a weak acid. It is based on the neutralization reaction, where. A known volume. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why It has a seriously complicated molecule which we will simplify to hlit. To do this you should add a small. These traits are desirable so only a small amount of an indicator is needed. Litmus is a weak acid. A known volume of the unknown concentration solution should be placed in a beaker under the burette. A useful indicator has. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why It is based on the neutralization reaction, where. Litmus is a weak acid. These traits are desirable so only a small amount of an indicator is needed. To do this you should add a small. The h is the proton which can be given. If a large amount of. A known volume of the unknown concentration solution should be placed. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why It is based on the neutralization reaction, where. It has a seriously complicated molecule which we will simplify to hlit. The h is the proton which can be given. A useful indicator has a strong color that changes quickly near its pka. If a large amount of. A known volume of the unknown concentration solution should be placed in a. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.numerade.com
SOLVEDQualitatively, describe how an acidbase indicator works. Why do The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why It is based on the neutralization reaction, where. Litmus is a weak acid. A known volume of the unknown concentration solution should be placed in a beaker under the burette. To do this you should add a small. It has a seriously complicated molecule which we will simplify to hlit. The h is the proton which can be given. A. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why A known volume of the unknown concentration solution should be placed in a beaker under the burette. These traits are desirable so only a small amount of an indicator is needed. Litmus is a weak acid. The h is the proton which can be given. A useful indicator has a strong color that changes quickly near its pka. If a. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From fyoqyaxpj.blob.core.windows.net
The Amount Of Indicator Used In An AcidBase Titration Must Be Small at The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why A known volume of the unknown concentration solution should be placed in a beaker under the burette. If a large amount of. To do this you should add a small. A useful indicator has a strong color that changes quickly near its pka. It is based on the neutralization reaction, where. Litmus is a weak acid. The h is the. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From mungfali.com
Acid Base Titration Calculation The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why To do this you should add a small. If a large amount of. A known volume of the unknown concentration solution should be placed in a beaker under the burette. Litmus is a weak acid. A useful indicator has a strong color that changes quickly near its pka. It has a seriously complicated molecule which we will simplify to hlit.. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why These traits are desirable so only a small amount of an indicator is needed. It is based on the neutralization reaction, where. A useful indicator has a strong color that changes quickly near its pka. A known volume of the unknown concentration solution should be placed in a beaker under the burette. Litmus is a weak acid. To do this. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why Litmus is a weak acid. The h is the proton which can be given. It is based on the neutralization reaction, where. A known volume of the unknown concentration solution should be placed in a beaker under the burette. To do this you should add a small. If a large amount of. It has a seriously complicated molecule which we. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why Litmus is a weak acid. If a large amount of. It is based on the neutralization reaction, where. These traits are desirable so only a small amount of an indicator is needed. A known volume of the unknown concentration solution should be placed in a beaker under the burette. A useful indicator has a strong color that changes quickly near. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why These traits are desirable so only a small amount of an indicator is needed. It has a seriously complicated molecule which we will simplify to hlit. If a large amount of. A known volume of the unknown concentration solution should be placed in a beaker under the burette. To do this you should add a small. The h is the. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.numerade.com
SOLVED bromothymol blue indicator is not a good indicator for strong The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why A known volume of the unknown concentration solution should be placed in a beaker under the burette. The h is the proton which can be given. To do this you should add a small. A useful indicator has a strong color that changes quickly near its pka. It has a seriously complicated molecule which we will simplify to hlit. It. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.sliderbase.com
Titration The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why These traits are desirable so only a small amount of an indicator is needed. If a large amount of. A known volume of the unknown concentration solution should be placed in a beaker under the burette. It is based on the neutralization reaction, where. To do this you should add a small. Litmus is a weak acid. A useful indicator. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From courses.lumenlearning.com
AcidBase Titrations Boundless Chemistry The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why It has a seriously complicated molecule which we will simplify to hlit. These traits are desirable so only a small amount of an indicator is needed. If a large amount of. A useful indicator has a strong color that changes quickly near its pka. To do this you should add a small. Litmus is a weak acid. A known volume. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why It is based on the neutralization reaction, where. These traits are desirable so only a small amount of an indicator is needed. To do this you should add a small. If a large amount of. The h is the proton which can be given. A known volume of the unknown concentration solution should be placed in a beaker under the. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.vecteezy.com
Acid base titration experiment and phases of color change during The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why A known volume of the unknown concentration solution should be placed in a beaker under the burette. If a large amount of. To do this you should add a small. It is based on the neutralization reaction, where. These traits are desirable so only a small amount of an indicator is needed. A useful indicator has a strong color that. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.numerade.com
SOLVEDThe amount of indicator used in an acidbase titration must be The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why If a large amount of. These traits are desirable so only a small amount of an indicator is needed. A useful indicator has a strong color that changes quickly near its pka. To do this you should add a small. The h is the proton which can be given. It is based on the neutralization reaction, where. Litmus is a. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.slideshare.net
Acid base titration The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why These traits are desirable so only a small amount of an indicator is needed. A known volume of the unknown concentration solution should be placed in a beaker under the burette. Litmus is a weak acid. It has a seriously complicated molecule which we will simplify to hlit. It is based on the neutralization reaction, where. If a large amount. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From fyoqyaxpj.blob.core.windows.net
The Amount Of Indicator Used In An AcidBase Titration Must Be Small at The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why To do this you should add a small. It has a seriously complicated molecule which we will simplify to hlit. A known volume of the unknown concentration solution should be placed in a beaker under the burette. These traits are desirable so only a small amount of an indicator is needed. A useful indicator has a strong color that changes. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why A useful indicator has a strong color that changes quickly near its pka. The h is the proton which can be given. If a large amount of. To do this you should add a small. These traits are desirable so only a small amount of an indicator is needed. It is based on the neutralization reaction, where. A known volume. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From www.science-revision.co.uk
Titrations The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why It is based on the neutralization reaction, where. It has a seriously complicated molecule which we will simplify to hlit. A known volume of the unknown concentration solution should be placed in a beaker under the burette. The h is the proton which can be given. Litmus is a weak acid. These traits are desirable so only a small amount. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why It has a seriously complicated molecule which we will simplify to hlit. A known volume of the unknown concentration solution should be placed in a beaker under the burette. If a large amount of. It is based on the neutralization reaction, where. The h is the proton which can be given. A useful indicator has a strong color that changes. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From mungfali.com
Acid Base Titration Indicator The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why If a large amount of. It is based on the neutralization reaction, where. To do this you should add a small. A useful indicator has a strong color that changes quickly near its pka. Litmus is a weak acid. The h is the proton which can be given. These traits are desirable so only a small amount of an indicator. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.
From courses.lumenlearning.com
AcidBase Titrations Chemistry The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why These traits are desirable so only a small amount of an indicator is needed. It is based on the neutralization reaction, where. A known volume of the unknown concentration solution should be placed in a beaker under the burette. It has a seriously complicated molecule which we will simplify to hlit. If a large amount of. A useful indicator has. The Amount Of Indicator Used In An Acid-Base Titration Must Be Small. Why.