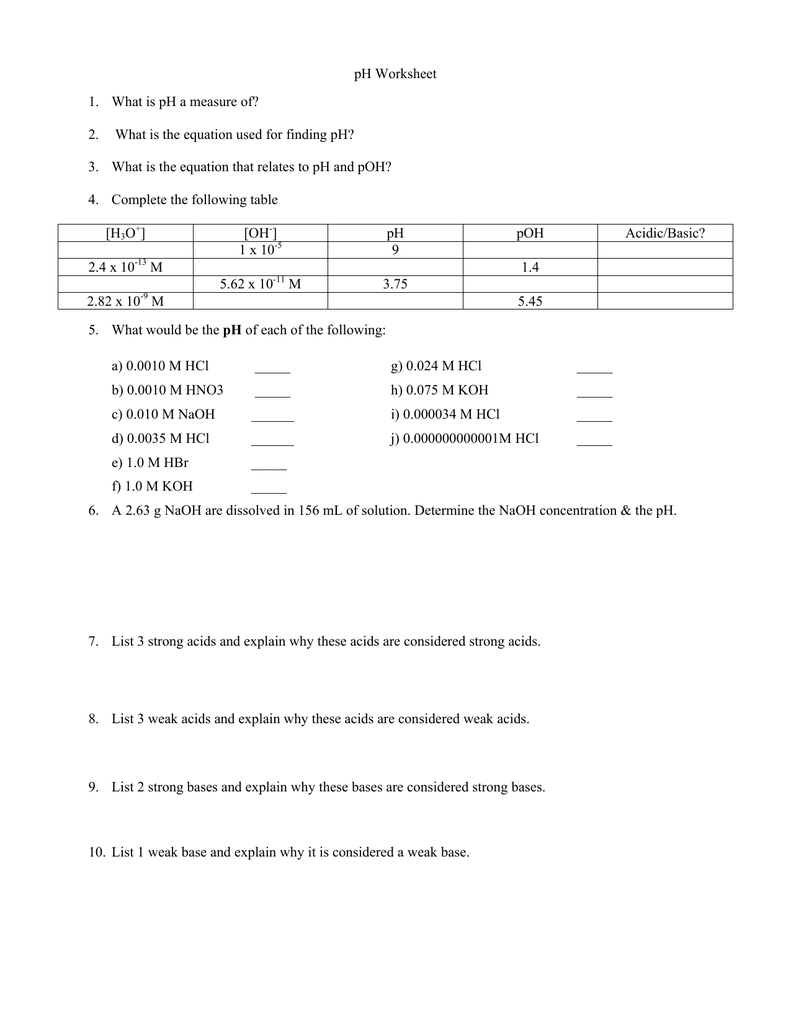
Ph Answer Key . Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. What is left in solution? The ph scale runs from 0 to 14,. The term “ph” is short for “potential of hydrogen.” it is a measure of how many excess h+ ions there are in a solution. For each of the problems below, assume. Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with ph, calculating the ph of strong. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. What is the concentration of [h+] in a solution whose ph = 4.3? We will calculate the ph of the solutions using the following 3 steps for each problem. Fill in the missing information in the table below. Poh is related to ph and can be easily calculated from ph.
from www.proworksheet.my.id
The term “ph” is short for “potential of hydrogen.” it is a measure of how many excess h+ ions there are in a solution. In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with ph, calculating the ph of strong. For each of the problems below, assume. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. What is left in solution? Fill in the missing information in the table below. What is the concentration of [h+] in a solution whose ph = 4.3? Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. The ph scale runs from 0 to 14,.
Ph Worksheet Answer Key
Ph Answer Key Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with ph, calculating the ph of strong. What is the concentration of [h+] in a solution whose ph = 4.3? Poh is related to ph and can be easily calculated from ph. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. What is left in solution? We will calculate the ph of the solutions using the following 3 steps for each problem. Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. For each of the problems below, assume. The ph scale runs from 0 to 14,. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. Fill in the missing information in the table below. The term “ph” is short for “potential of hydrogen.” it is a measure of how many excess h+ ions there are in a solution.
From quizzcampusparis123.z21.web.core.windows.net
Ph And Poh Worksheet Answer Key Ph Answer Key Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. For each of the problems below, assume. Fill in the missing information in the table below. The ph scale runs from 0 to 14,. We will calculate the ph of the solutions using the following 3. Ph Answer Key.
From studylib.net
pH Scale Basics Remote Lab1 Ph Answer Key What is the concentration of [h+] in a solution whose ph = 4.3? Poh is related to ph and can be easily calculated from ph. Fill in the missing information in the table below. The ph scale runs from 0 to 14,. The term “ph” is short for “potential of hydrogen.” it is a measure of how many excess h+. Ph Answer Key.
From studylib.net
pH Math Practice (Chapter 3) ANSWERS Complete the following Ph Answer Key Poh is related to ph and can be easily calculated from ph. What is the concentration of [h+] in a solution whose ph = 4.3? What is left in solution? For each of the problems below, assume. The ph scale runs from 0 to 14,. In this set of practice problems, we will work on examples correlating the acidity and. Ph Answer Key.
From www.proworksheet.my.id
Ph Worksheet Answer Key Ph Answer Key For each of the problems below, assume. The term “ph” is short for “potential of hydrogen.” it is a measure of how many excess h+ ions there are in a solution. What is left in solution? The ph scale runs from 0 to 14,. Poh is related to ph and can be easily calculated from ph. In this set of. Ph Answer Key.
From www.tes.com
Acids, Bases, and the pH Scale Worksheet Printable and Distance Ph Answer Key Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. The term “ph” is short for “potential of hydrogen.” it is a measure of how many excess h+ ions there are in a solution. We will calculate the ph of the solutions using the following 3. Ph Answer Key.
From materialzonekonner88.z21.web.core.windows.net
Ph Worksheet Answer Key Ph Answer Key For each of the problems below, assume. Poh is related to ph and can be easily calculated from ph. What is the concentration of [h+] in a solution whose ph = 4.3? The ph scale runs from 0 to 14,. In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with. Ph Answer Key.
From studylibackermann.z19.web.core.windows.net
Ph Worksheet 1 Answer Key Ph Answer Key Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. For each of the problems below, assume. In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with ph, calculating the ph of strong. The term “ph” is short for “potential of hydrogen.” it. Ph Answer Key.
From keepnotes.com
pH Analysis Gizmo Answer Key Virtual High School KeepNotes Ph Answer Key Poh is related to ph and can be easily calculated from ph. In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with ph, calculating the ph of strong. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. We will calculate the ph. Ph Answer Key.
From www.studocu.com
Matthew Chan Calculating p H Name Matthew Chan Calculating pH How is Ph Answer Key What is the ph of a solution that has a hydronium concentration of 3.4 x 10. In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with ph, calculating the ph of strong. Fill in the missing information in the table below. What is left in solution? The term “ph” is. Ph Answer Key.
From www.chegg.com
Solved NAME PER CHEMISTRY pH WORKSHEET pHPOH[H][OH] 1. Ph Answer Key In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with ph, calculating the ph of strong. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. Using the examples given in model 3, write a sentence or a mathematical equation that describes how. Ph Answer Key.
From www.e-streetlight.com
Ph Worksheet Answer Key E Street Light Ph Answer Key The term “ph” is short for “potential of hydrogen.” it is a measure of how many excess h+ ions there are in a solution. Poh is related to ph and can be easily calculated from ph. In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with ph, calculating the ph. Ph Answer Key.
From dbdalrympleconfines.z21.web.core.windows.net
Acids/bases And Ph Worksheet Ph Answer Key The ph scale runs from 0 to 14,. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. What is left in solution? Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. Ph and poh are defined as the. Ph Answer Key.
From www.studypool.com
SOLUTION Ph poh ws key Studypool Ph Answer Key What is left in solution? Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. For each of the problems below, assume. Fill in the missing information in the table below. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. The ph scale runs from 0. Ph Answer Key.
From studylib.net
Phet pH Scale Basics Worksheet Ph Answer Key The ph scale runs from 0 to 14,. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. We will calculate the ph of the solutions using the following 3 steps for each problem. In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution. Ph Answer Key.
From studyschoolpfaff.z19.web.core.windows.net
Acids Bases & Ph Worksheets Answer Key Ph Answer Key The ph scale runs from 0 to 14,. What is left in solution? We will calculate the ph of the solutions using the following 3 steps for each problem. Fill in the missing information in the table below. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. Ph and poh are defined as. Ph Answer Key.
From worksheetlistlemann.z13.web.core.windows.net
Acids Bases And The Ph Scale Worksheets Answer Key Ph Answer Key In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with ph, calculating the ph of strong. Fill in the missing information in the table below. What is the concentration of [h+] in a solution whose ph = 4.3? Ph and poh are defined as the negative log of hydrogen ion. Ph Answer Key.
From printablelibbrained.z13.web.core.windows.net
Investigating The Ph Scale Answer Key Ph Answer Key The ph scale runs from 0 to 14,. For each of the problems below, assume. Fill in the missing information in the table below. What is left in solution? Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. The term “ph” is short for “potential. Ph Answer Key.
From www.proworksheet.my.id
Ph Worksheet Answer Key Ph Answer Key For each of the problems below, assume. Fill in the missing information in the table below. The term “ph” is short for “potential of hydrogen.” it is a measure of how many excess h+ ions there are in a solution. The ph scale runs from 0 to 14,. What is the concentration of [h+] in a solution whose ph =. Ph Answer Key.
From www.chemistrylearner.com
Free Printable pH and pOH Worksheets Ph Answer Key What is the concentration of [h+] in a solution whose ph = 4.3? Fill in the missing information in the table below. Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. We will calculate the ph of the solutions using the following 3 steps for. Ph Answer Key.
From www.proworksheet.my.id
Ph Worksheet Answer Key Ph Answer Key What is the ph of a solution that has a hydronium concentration of 3.4 x 10. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. The ph scale. Ph Answer Key.
From studylibackermann.z19.web.core.windows.net
Ph Worksheet 1 Answer Key Ph Answer Key Fill in the missing information in the table below. Poh is related to ph and can be easily calculated from ph. We will calculate the ph of the solutions using the following 3 steps for each problem. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. What is left in solution? For each. Ph Answer Key.
From printablezoneexpose.z13.web.core.windows.net
Chemistry Ph Worksheet Answers Ph Answer Key For each of the problems below, assume. We will calculate the ph of the solutions using the following 3 steps for each problem. Poh is related to ph and can be easily calculated from ph. In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with ph, calculating the ph of. Ph Answer Key.
From www.studocu.com
PH Analysis in chemistry 2 Lab Gizmo Answers 2019 Name Ph Answer Key Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. We will calculate the ph of the solutions using the following 3 steps for each problem. The term “ph” is short for “potential of hydrogen.” it is a measure of how many excess h+ ions there. Ph Answer Key.
From www.pdffiller.com
Brainpop Ph Scale Worksheet Answers Fill Online, Printable, Fillable Ph Answer Key Fill in the missing information in the table below. What is the concentration of [h+] in a solution whose ph = 4.3? The ph scale runs from 0 to 14,. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. Using the examples given in model 3, write a sentence or a mathematical equation. Ph Answer Key.
From printablecampusfisher.z19.web.core.windows.net
Ph And Poh Calculations Worksheet Answer Key Ph Answer Key Fill in the missing information in the table below. The ph scale runs from 0 to 14,. What is the concentration of [h+] in a solution whose ph = 4.3? The term “ph” is short for “potential of hydrogen.” it is a measure of how many excess h+ ions there are in a solution. What is the ph of a. Ph Answer Key.
From studylib.net
Chem 12 WS4.5 (pH and pOH) Ph Answer Key Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. We will calculate the ph of the solutions using the following 3 steps for each problem. What is left in solution? Poh is related to ph and can be easily calculated from ph. Using the examples given in model 3, write a sentence. Ph Answer Key.
From www.tes.com
Acids, Bases, and the pH Scale Worksheet Printable and Distance Ph Answer Key We will calculate the ph of the solutions using the following 3 steps for each problem. For each of the problems below, assume. The ph scale runs from 0 to 14,. Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. What is the ph of. Ph Answer Key.
From quizzmediacowan.z21.web.core.windows.net
Ph Calculations Worksheet Answers Key With Work Ph Answer Key Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. Fill in the missing information in the table below. What is the concentration of [h+] in a solution whose ph = 4.3? What is left in solution? The ph scale runs from 0 to 14,. For. Ph Answer Key.
From lessonschoolsheepos.z14.web.core.windows.net
Ph Calculations Worksheet Answers Key With Work Ph Answer Key In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with ph, calculating the ph of strong. For each of the problems below, assume. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. What is the concentration of [h+] in a solution whose. Ph Answer Key.
From printableella99.z21.web.core.windows.net
The Ph Scale Worksheet Answer Key Ph Answer Key Fill in the missing information in the table below. What is left in solution? Poh is related to ph and can be easily calculated from ph. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. Using the examples given in model 3, write a sentence or a mathematical equation that describes how to. Ph Answer Key.
From classschooltrommler.z19.web.core.windows.net
Ph Calculations Practice Worksheet Answers Ph Answer Key What is the ph of a solution that has a hydronium concentration of 3.4 x 10. Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with. Ph Answer Key.
From keepnotes.com
pH Analysis Gizmo Answer Key Virtual High School KeepNotes Ph Answer Key What is left in solution? Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. The term “ph” is short for “potential of hydrogen.” it is a measure of how many excess h+ ions there are in a solution. What is the concentration of [h+] in. Ph Answer Key.
From www.transtutors.com
(Solved) REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A Ph Answer Key What is the concentration of [h+] in a solution whose ph = 4.3? Poh is related to ph and can be easily calculated from ph. What is left in solution? The ph scale runs from 0 to 14,. In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with ph, calculating. Ph Answer Key.
From studylib.net
Acids and Bases Answer Key Ph Answer Key What is the concentration of [h+] in a solution whose ph = 4.3? In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with ph, calculating the ph of strong. Poh is related to ph and can be easily calculated from ph. Using the examples given in model 3, write a. Ph Answer Key.
From www.e-streetlight.com
Ph Worksheet Answer Key E Street Light Ph Answer Key What is the ph of a solution that has a hydronium concentration of 3.4 x 10. Fill in the missing information in the table below. What is left in solution? Using the examples given in model 3, write a sentence or a mathematical equation that describes how to calculate ph from the hydronium ion. In this set of practice problems,. Ph Answer Key.