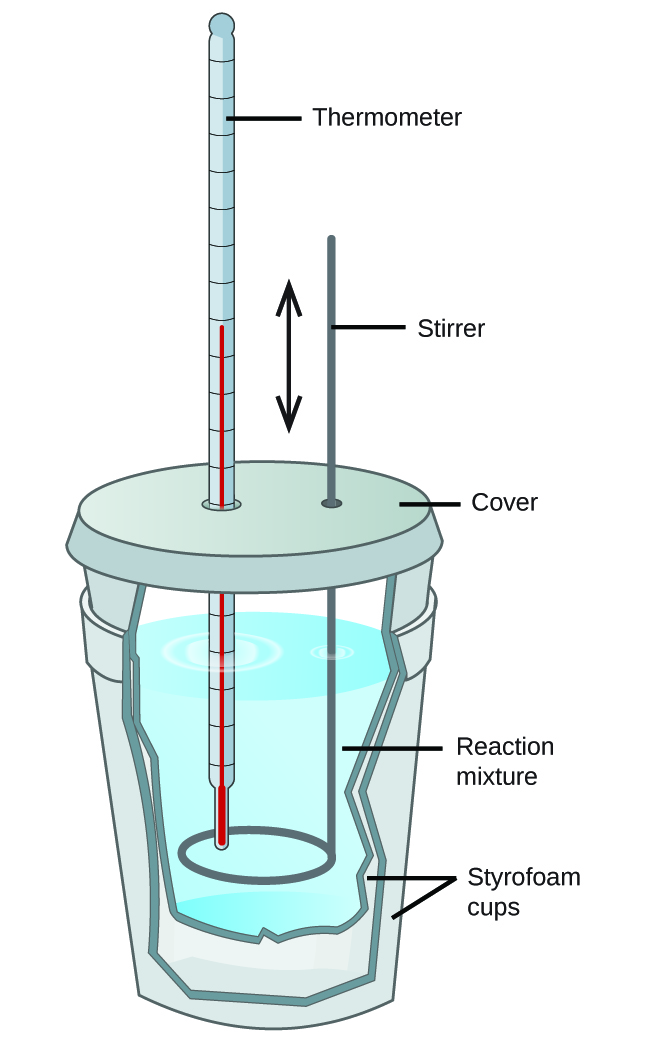
Calorimeter Definition And Significance . — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. The amount of heat released or absorbed. — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The process of measuring this heat is called. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state.
from ecampusontario.pressbooks.pub
The process of measuring this heat is called. — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. The amount of heat released or absorbed. — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
5.2 Calorimetry Chemistry
Calorimeter Definition And Significance — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The amount of heat released or absorbed. — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The process of measuring this heat is called.
From www.slideserve.com
PPT a “ Calorimeter ” PowerPoint Presentation, free download ID7050684 Calorimeter Definition And Significance — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. The process of measuring this heat is called. — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — a calorimeter is a device used to measure the amount of. Calorimeter Definition And Significance.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Definition And Significance The amount of heat released or absorbed. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. — calorimeter, device for measuring the heat developed during a mechanical, electrical,. Calorimeter Definition And Significance.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimeter Definition And Significance The process of measuring this heat is called. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. The amount of heat released or absorbed. —. Calorimeter Definition And Significance.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab Calorimeter Definition And Significance — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The amount of heat released or absorbed. — calorimeter, device for measuring the heat developed during a mechanical,. Calorimeter Definition And Significance.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter Definition And Significance The amount of heat released or absorbed. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The process of measuring this heat is called. — calorimetry is the science. Calorimeter Definition And Significance.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimeter Definition And Significance — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. The process of measuring this heat is called. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. — calorimetry is the science of measuring the heat exchange between a system and its surroundings to. Calorimeter Definition And Significance.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter Definition And Significance — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. The amount of heat released or absorbed. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. — a calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimeter Definition And Significance.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter Definition And Significance — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The process of measuring this heat is called. — a calorimeter is a device used to measure the heat flow of a chemical. Calorimeter Definition And Significance.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Definition And Significance The amount of heat released or absorbed. The process of measuring this heat is called. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. — calorimeter, device for measuring. Calorimeter Definition And Significance.
From exopfwwvc.blob.core.windows.net
Calorimeter In Physics Definition at Laura Addy blog Calorimeter Definition And Significance — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. The process of measuring this heat is called. The amount of heat released or absorbed. . Calorimeter Definition And Significance.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Calorimeter Definition And Significance — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The amount of heat released. Calorimeter Definition And Significance.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Definition And Significance The amount of heat released or absorbed. — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. The process of measuring this heat is called. — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. — calorimetry is the measurement. Calorimeter Definition And Significance.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter Definition And Significance — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. The process of measuring this heat is called. — calorimeter, device for measuring the heat developed during a mechanical,. Calorimeter Definition And Significance.
From www.animalia-life.club
Calorimeter Diagram Calorimeter Definition And Significance The process of measuring this heat is called. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. — calorimeter, device for measuring the heat developed during a. Calorimeter Definition And Significance.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Calorimeter Definition And Significance — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. — calorimetry is. Calorimeter Definition And Significance.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimeter Definition And Significance The process of measuring this heat is called. — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The amount of heat released or absorbed. — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — calorimetry is the measurement of the heat involved. Calorimeter Definition And Significance.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Calorimeter Definition And Significance — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. The amount of heat released or absorbed. The process of measuring this heat is called. — a calorimeter is a device used to measure the. Calorimeter Definition And Significance.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Definition And Significance — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. The process of measuring this heat is called. The amount of heat released or absorbed. — a calorimeter is a device used to measure the. Calorimeter Definition And Significance.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Definition And Significance — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. — calorimetry is. Calorimeter Definition And Significance.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Definition And Significance — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. — calorimetry is the measurement of the heat involved in a chemical reaction or. Calorimeter Definition And Significance.
From testbook.com
Calorimeter Definition, Diagram, Working, Types, Applications Calorimeter Definition And Significance The process of measuring this heat is called. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. — a calorimeter is a device used to measure the amount of. Calorimeter Definition And Significance.
From studylib.net
Calorimetry Calorimeter Definition And Significance — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. The process of measuring this heat is called. The amount of heat released or absorbed. — calorimetry is the. Calorimeter Definition And Significance.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Calorimeter Definition And Significance — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. The process of measuring this heat is called. — a calorimeter is a device used to measure the amount of. Calorimeter Definition And Significance.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Calorimeter Definition And Significance — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. — a calorimeter is a device used to measure the amount of heat involved in. Calorimeter Definition And Significance.
From www.scribd.com
Calorimeter and Its Types Continuum Mechanics Branches Of Calorimeter Definition And Significance — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The amount of heat released or absorbed. The process of measuring this heat is called. — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of.. Calorimeter Definition And Significance.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter Definition And Significance — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — calorimetry is. Calorimeter Definition And Significance.
From gamesmartz.com
Calorimeter Definition Easy to Understand Calorimeter Definition And Significance — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The process of measuring this heat is called. — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — a calorimeter is a device used to measure the amount of heat involved in a. Calorimeter Definition And Significance.
From exopfwwvc.blob.core.windows.net
Calorimeter In Physics Definition at Laura Addy blog Calorimeter Definition And Significance — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. The amount of heat released or absorbed. The process of measuring this heat is called. — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. —. Calorimeter Definition And Significance.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter Definition And Significance — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The process of measuring this heat is called. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. — calorimeter, device for measuring the heat developed during a mechanical,. Calorimeter Definition And Significance.
From study.com
Calorimetry Definition, Equation & Types Lesson Calorimeter Definition And Significance — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. The process of measuring this heat is called. — a calorimeter is a device used to measure the heat flow of a chemical reaction or. Calorimeter Definition And Significance.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Definition And Significance — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The process of measuring this heat is called. The amount of heat released or absorbed. — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. — calorimetry is. Calorimeter Definition And Significance.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Calorimeter Definition And Significance — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. —. Calorimeter Definition And Significance.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Calorimeter Definition And Significance — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. — calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. — calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. —. Calorimeter Definition And Significance.
From ecampusontario.pressbooks.pub
5.2 Calorimetry Chemistry Calorimeter Definition And Significance The process of measuring this heat is called. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. — a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. — calorimeter, device for measuring the heat developed during a. Calorimeter Definition And Significance.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Definition And Significance The amount of heat released or absorbed. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. — calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of. — a calorimeter is a device used. Calorimeter Definition And Significance.