Dilution Si Unit . serial dilution is a process by which a series of solutions can be made which conserves the amount of chemical needed. — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. [2] however, values are often quoted in s cm. the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. For example, a 1:4 dilution. — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of a solution in measured in. the si unit of molar conductivity is siemens metres squared per mole (s m 2 mol −1).
from ecampusontario.pressbooks.pub
the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. serial dilution is a process by which a series of solutions can be made which conserves the amount of chemical needed. the si unit of molar conductivity is siemens metres squared per mole (s m 2 mol −1). For example, a 1:4 dilution. — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). [2] however, values are often quoted in s cm. — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of a solution in measured in.
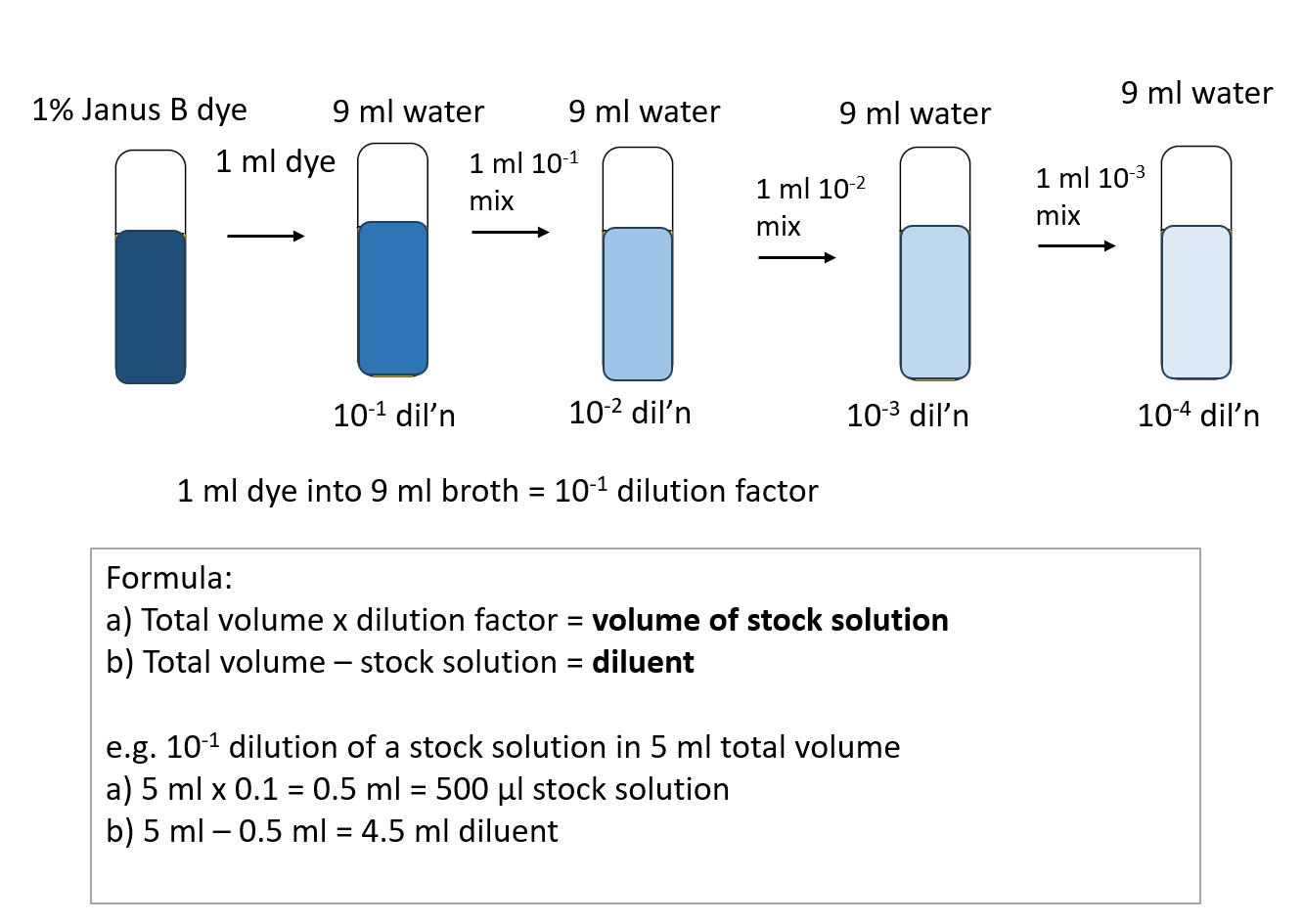
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual
Dilution Si Unit For example, a 1:4 dilution. serial dilution is a process by which a series of solutions can be made which conserves the amount of chemical needed. the si unit of molar conductivity is siemens metres squared per mole (s m 2 mol −1). [2] however, values are often quoted in s cm. For example, a 1:4 dilution. the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of a solution in measured in. — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d).
From www.pdfnotes.co
All SI Units List PDF Dilution Si Unit — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of a solution in measured. Dilution Si Unit.
From exovmtirc.blob.core.windows.net
Dilution Vs. Absorption at Charlie Goings blog Dilution Si Unit — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). [2] however, values are often quoted in s cm. serial dilution is a process by which a series of solutions can be. Dilution Si Unit.
From www.wolframalpha.com
Dilution Calculator WolframAlpha Chemistry Solvers Dilution Si Unit — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). For example, a 1:4 dilution. the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. serial dilution is a process by. Dilution Si Unit.
From thestudentnotes.com
Serial Dilution Definition, Formula, Calculator, Procedure, Uses The Dilution Si Unit serial dilution is a process by which a series of solutions can be made which conserves the amount of chemical needed. — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. the si unit of molar conductivity is siemens metres squared per mole (s m 2 mol −1). . Dilution Si Unit.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Si Unit the si unit of molar conductivity is siemens metres squared per mole (s m 2 mol −1). serial dilution is a process by which a series of solutions can be made which conserves the amount of chemical needed. For example, a 1:4 dilution. — the dilution ratio tells us the amount of solute compared to the amount. Dilution Si Unit.
From www.doubtnut.com
Use of dilution formula (M(1)V(1) = M(2) V(2)) Dilution Si Unit — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. serial dilution is a process by which a series of solutions. Dilution Si Unit.
From spmchemistry.blog.onlinetuition.com.my
Dilution SPM Chemistry Dilution Si Unit the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. [2] however, values are often quoted in s cm. — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). — diluting. Dilution Si Unit.
From www.youtube.com
Unit 4 09 Dilution of Solutions YouTube Dilution Si Unit For example, a 1:4 dilution. — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of a solution in measured in. — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. the formula for a dilution is v1 x c1 = v2. Dilution Si Unit.
From www.studocu.com
Mol Bio Worksheet Theory Dna Techniques SI Unit Prefixes (m Dilution Si Unit For example, a 1:4 dilution. [2] however, values are often quoted in s cm. the si unit of molar conductivity is siemens metres squared per mole (s m 2 mol −1). — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of a solution in measured in. serial dilution is. Dilution Si Unit.
From www.youtube.com
Microbiology Serial Dilution calculation YouTube Dilution Si Unit — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). For example, a 1:4 dilution. — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2. Dilution Si Unit.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Si Unit the si unit of molar conductivity is siemens metres squared per mole (s m 2 mol −1). For example, a 1:4 dilution. the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. serial dilution is a process by. Dilution Si Unit.
From www.dynamicscience.com.au
Chemistry dilution Dilution Si Unit — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. [2] however, values are often quoted in s cm. serial. Dilution Si Unit.
From bio.libretexts.org
15 Determination of Bacterial Numbers Biology LibreTexts Dilution Si Unit — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. For example, a 1:4 dilution. — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of a solution in measured in. serial dilution is a process by which a series of solutions. Dilution Si Unit.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilution Si Unit serial dilution is a process by which a series of solutions can be made which conserves the amount of chemical needed. — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. . Dilution Si Unit.
From labrobot.com
Decimal dilutions Food microbiology Dilucup System Dilution Si Unit — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). For example, a 1:4 dilution. — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of a solution in measured in. serial dilution is a process by which a series of solutions can. Dilution Si Unit.
From www.youtube.com
Serial Dilution Technique For Microbiological & Chemical Analysis Dilution Si Unit [2] however, values are often quoted in s cm. the si unit of molar conductivity is siemens metres squared per mole (s m 2 mol −1). serial dilution is a process by which a series of solutions can be made which conserves the amount of chemical needed. — the dilution ratio tells us the amount of solute. Dilution Si Unit.
From www.wolframalpha.com
Dilution Calculator WolframAlpha Chemistry Solvers Dilution Si Unit — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. [2] however, values are often quoted in s cm. — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of a solution in measured in. For example, a 1:4 dilution. — the. Dilution Si Unit.
From www.pinterest.com
Units of Concentration Should've probably printed this for Dilution Si Unit [2] however, values are often quoted in s cm. the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). serial dilution. Dilution Si Unit.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Si Unit [2] however, values are often quoted in s cm. the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. For example, a 1:4 dilution. — the dilution ratio tells us the amount of solute compared to the amount of. Dilution Si Unit.
From www.scribd.com
Dilution Video Handout PDF Concentration Colony Forming Unit Dilution Si Unit For example, a 1:4 dilution. — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of a solution in measured in. the formula for a dilution is v1 x c1 = v2. Dilution Si Unit.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Si Unit — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). the si unit of molar conductivity is siemens metres squared per mole (s m 2 mol −1). the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after. Dilution Si Unit.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Si Unit [2] however, values are often quoted in s cm. — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. the si unit of molar conductivity is siemens metres squared per mole (s m 2 mol −1). — moreover, since the density of dilute aqueous solutions are close to 1. Dilution Si Unit.
From dxobhpmpa.blob.core.windows.net
Dilution Mass Per Volume at Thomas Bowling blog Dilution Si Unit — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. [2] however, values are often quoted in s cm. the si. Dilution Si Unit.
From hereditybio.in
Serial Dilution Technique for Bacterial Quantification Procedure and Dilution Si Unit — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of a solution in measured in. For example, a 1:4 dilution. the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. . Dilution Si Unit.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions Dilution Si Unit the si unit of molar conductivity is siemens metres squared per mole (s m 2 mol −1). serial dilution is a process by which a series of solutions can be made which conserves the amount of chemical needed. the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the. Dilution Si Unit.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Dilution Si Unit — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. the si unit of molar conductivity is siemens metres squared per. Dilution Si Unit.
From www.tffn.net
What is an SI Unit in Science? Exploring the Basics and Benefits of Dilution Si Unit serial dilution is a process by which a series of solutions can be made which conserves the amount of chemical needed. — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of. Dilution Si Unit.
From www.schoolmouv.fr
Dilution et dissolution Fiche de cours Physiquechimie SchoolMouv Dilution Si Unit the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. serial dilution is a process by which a series of solutions can be made which conserves the amount of chemical needed. [2] however, values are often quoted in s. Dilution Si Unit.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution Si Unit — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. [2] however, values are often quoted in s cm. For example, a 1:4 dilution. the si unit of molar conductivity is siemens. Dilution Si Unit.
From chem370.github.io
Lab 1 Prelab CHEM 370 Dilution Si Unit — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. the si unit of molar conductivity is siemens metres squared per mole (s m 2 mol −1). [2] however, values are often quoted in s cm. serial dilution is a process by which a series of solutions can be. Dilution Si Unit.
From www.studocu.com
Dilutions and concentrations answers SI Units and concentrations Dilution Si Unit — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of a solution in measured in. For example, a 1:4 dilution. the formula for a dilution is v1 x c1 = v2 x. Dilution Si Unit.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Si Unit For example, a 1:4 dilution. the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. — the dilution ratio tells us the amount of solute compared to the amount of solvent (s:d). the si unit of molar conductivity. Dilution Si Unit.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Si Unit the formula for a dilution is v1 x c1 = v2 x c2 (where c1 and c2 are the concentrations before and after dilution, and v1 and v2 are. serial dilution is a process by which a series of solutions can be made which conserves the amount of chemical needed. — moreover, since the density of dilute. Dilution Si Unit.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual Dilution Si Unit — diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is. [2] however, values are often quoted in s cm. For example, a 1:4 dilution. the si unit of molar conductivity is siemens metres squared per mole (s m 2 mol −1). serial dilution is a process by which a. Dilution Si Unit.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Si Unit serial dilution is a process by which a series of solutions can be made which conserves the amount of chemical needed. [2] however, values are often quoted in s cm. — moreover, since the density of dilute aqueous solutions are close to 1 g/ml, if the volume of a solution in measured in. the formula for a. Dilution Si Unit.