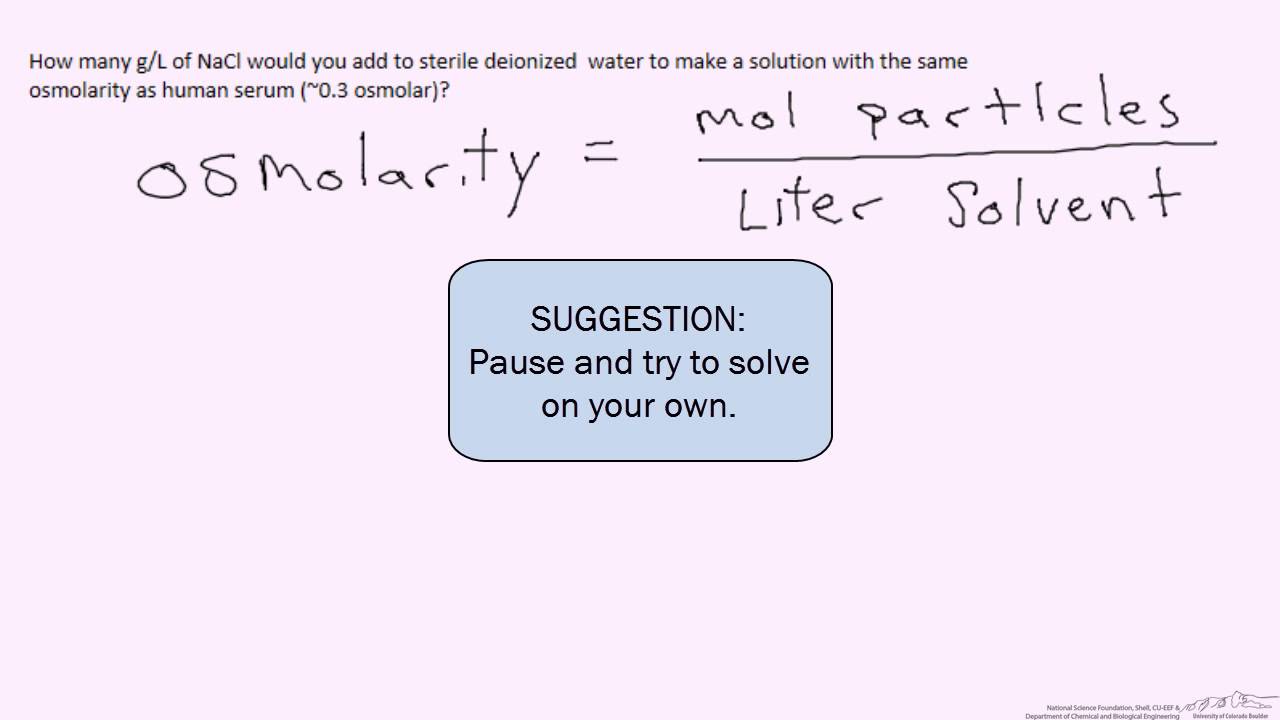
How To Determine Osmolality Of A Solution . An osmole (osmol) is 1 mol of particles that. Osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of water across cell. The osmolarity of solutions containing a single type of solute (for example: Osmolarity is the number of osmoles of solute per litre of solution. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. You multiply the molarity by the number of osmoles that each solute produces. Convert that number to osmoles per liter and add the osmolarity of each. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. How do you calculate osmolarity? An osmole is 1 mol of particles that contribute to the. Just glucose or just sodium.
from www.youtube.com
An osmole is 1 mol of particles that contribute to the. How do you calculate osmolarity? Osmolarity is the number of osmoles of solute per litre of solution. The osmolarity of solutions containing a single type of solute (for example: Just glucose or just sodium. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): Osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of water across cell. An osmole (osmol) is 1 mol of particles that. Convert that number to osmoles per liter and add the osmolarity of each.
Osmolarity Example (Bio) YouTube
How To Determine Osmolality Of A Solution Convert that number to osmoles per liter and add the osmolarity of each. Convert that number to osmoles per liter and add the osmolarity of each. An osmole is 1 mol of particles that contribute to the. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): The osmolarity of solutions containing a single type of solute (for example: Osmolarity is the number of osmoles of solute per litre of solution. Just glucose or just sodium. An osmole (osmol) is 1 mol of particles that. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. How do you calculate osmolarity? Osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of water across cell. You multiply the molarity by the number of osmoles that each solute produces.
From ojklmexhbr.blogspot.com
How To Calculate Serum Osmolality In this study, we compared the How To Determine Osmolality Of A Solution The osmolarity of solutions containing a single type of solute (for example: You multiply the molarity by the number of osmoles that each solute produces. An osmole (osmol) is 1 mol of particles that. How do you calculate osmolarity? To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter. How To Determine Osmolality Of A Solution.
From www.slideserve.com
PPT Water Metabolism PowerPoint Presentation, free download ID4493121 How To Determine Osmolality Of A Solution How do you calculate osmolarity? To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): An osmole is 1 mol of particles that contribute to the. The osmolarity. How To Determine Osmolality Of A Solution.
From exonkuxev.blob.core.windows.net
Define Stool Osmolality at Christine Poyner blog How To Determine Osmolality Of A Solution Convert that number to osmoles per liter and add the osmolarity of each. An osmole is 1 mol of particles that contribute to the. You multiply the molarity by the number of osmoles that each solute produces. How do you calculate osmolarity? To calculate the osmolarity of a solution, the first step is to convert the number to moles per. How To Determine Osmolality Of A Solution.
From www.youtube.com
Calculate your own osmolarity Lab values and concentrations Health How To Determine Osmolality Of A Solution An osmole is 1 mol of particles that contribute to the. You multiply the molarity by the number of osmoles that each solute produces. Convert that number to osmoles per liter and add the osmolarity of each. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): How do you. How To Determine Osmolality Of A Solution.
From fyoplrsjt.blob.core.windows.net
How Do You Estimate Osmolarity at Pauline Holman blog How To Determine Osmolality Of A Solution To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): The osmolarity of solutions containing a single type of solute (for example: To calculate the osmolarity of a. How To Determine Osmolality Of A Solution.
From www.chegg.com
Solved How can cells adjust to the osmolality of their How To Determine Osmolality Of A Solution Just glucose or just sodium. How do you calculate osmolarity? Osmolarity is the number of osmoles of solute per litre of solution. Convert that number to osmoles per liter and add the osmolarity of each. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. Indeed, the osmolality. How To Determine Osmolality Of A Solution.
From pediaa.com
Difference Between Osmolarity and Osmolality Definition, Explanation How To Determine Osmolality Of A Solution Convert that number to osmoles per liter and add the osmolarity of each. An osmole is 1 mol of particles that contribute to the. Osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of water across cell. To calculate the osmolarity of a solution, the first step is to convert the number. How To Determine Osmolality Of A Solution.
From www.slideserve.com
PPT Diagnostic Tests PowerPoint Presentation ID210922 How To Determine Osmolality Of A Solution How do you calculate osmolarity? Convert that number to osmoles per liter and add the osmolarity of each. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf):. How To Determine Osmolality Of A Solution.
From www.slideserve.com
PPT ENTERAL NUTRITION PowerPoint Presentation ID225635 How To Determine Osmolality Of A Solution Convert that number to osmoles per liter and add the osmolarity of each. Osmolarity is the number of osmoles of solute per litre of solution. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. Indeed, the osmolality of a solution is typically determined most accurately and conveniently. How To Determine Osmolality Of A Solution.
From www.youtube.com
How to solve osmolarity calculation problems 4 YouTube How To Determine Osmolality Of A Solution Osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of water across cell. An osmole (osmol) is 1 mol of particles that. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): Just glucose or just sodium. Convert that number to osmoles. How To Determine Osmolality Of A Solution.
From www.pinterest.com
Osmolality Physiology, Medicine, Concentration How To Determine Osmolality Of A Solution Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. How do you calculate osmolarity? Osmolarity is the number of osmoles of solute per litre of solution. An osmole (osmol) is. How To Determine Osmolality Of A Solution.
From ojklmexhbr.blogspot.com
How To Calculate Serum Osmolality In this study, we compared the How To Determine Osmolality Of A Solution An osmole is 1 mol of particles that contribute to the. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. An osmole (osmol) is 1 mol of particles that. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter. How To Determine Osmolality Of A Solution.
From www.solutionspile.com
[Solved] A homogeneous mixture, ( 600.00 mathrm{mL} ) How To Determine Osmolality Of A Solution An osmole (osmol) is 1 mol of particles that. The osmolarity of solutions containing a single type of solute (for example: How do you calculate osmolarity? You multiply the molarity by the number of osmoles that each solute produces. Just glucose or just sodium. An osmole is 1 mol of particles that contribute to the. To calculate the osmolarity in. How To Determine Osmolality Of A Solution.
From www.youtube.com
Osmolarity Example (Bio) YouTube How To Determine Osmolality Of A Solution To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. Just glucose or just sodium. Osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of water across cell. An osmole (osmol) is 1 mol of particles that. Osmolarity is the. How To Determine Osmolality Of A Solution.
From droualb.faculty.mjc.edu
Chapter 4 How To Determine Osmolality Of A Solution To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. An osmole (osmol) is 1 mol of particles that. You multiply the molarity by the number of osmoles that each solute produces. Just glucose or just sodium. To calculate the osmolarity of a solution, the first step is. How To Determine Osmolality Of A Solution.
From www.youtube.com
Calculate Molecular Weight from Osmotic Pressure for Nonideal Solution How To Determine Osmolality Of A Solution Osmolarity is the number of osmoles of solute per litre of solution. You multiply the molarity by the number of osmoles that each solute produces. Just glucose or just sodium. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. How do you calculate osmolarity? An osmole is. How To Determine Osmolality Of A Solution.
From www.researchgate.net
Detachment under changes in solution composition, osmolality, and How To Determine Osmolality Of A Solution An osmole is 1 mol of particles that contribute to the. Just glucose or just sodium. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): An osmole (osmol) is 1 mol of particles that. How do you calculate osmolarity? You multiply the molarity by the number of osmoles that. How To Determine Osmolality Of A Solution.
From www.pinterest.com
Osmolality It is, Search and Ranges How To Determine Osmolality Of A Solution To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. An osmole is 1 mol of particles that contribute to the. Convert that number to osmoles per liter and add the osmolarity of each. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by. How To Determine Osmolality Of A Solution.
From www.slideserve.com
PPT Clinical Chemistry Chapter 4 PowerPoint Presentation, free How To Determine Osmolality Of A Solution Osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of water across cell. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. Convert that number to osmoles per liter and add the osmolarity of each. Indeed, the osmolality of. How To Determine Osmolality Of A Solution.
From fyotxaglo.blob.core.windows.net
How To Find Osmolality Of Solution at Villacorta blog How To Determine Osmolality Of A Solution The osmolarity of solutions containing a single type of solute (for example: To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. An osmole is 1 mol of particles that contribute to the. Just glucose or just sodium. How do you calculate osmolarity? You multiply the molarity by. How To Determine Osmolality Of A Solution.
From coggle.it
1 Cell Biology, 1.1 Intro to Cells (Cell Size (Limited by its need to… How To Determine Osmolality Of A Solution How do you calculate osmolarity? Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): Just glucose or just sodium. Osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of water across cell. An osmole (osmol) is 1 mol of particles that.. How To Determine Osmolality Of A Solution.
From www.chegg.com
Solved How can cells adjust to the osmolality of their How To Determine Osmolality Of A Solution Convert that number to osmoles per liter and add the osmolarity of each. An osmole is 1 mol of particles that contribute to the. The osmolarity of solutions containing a single type of solute (for example: To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. You multiply the molarity by. How To Determine Osmolality Of A Solution.
From aneskey.com
Osmolality, sodium, potassium, chloride, and bicarbonate Anesthesia Key How To Determine Osmolality Of A Solution You multiply the molarity by the number of osmoles that each solute produces. An osmole (osmol) is 1 mol of particles that. Osmolarity is the number of osmoles of solute per litre of solution. Just glucose or just sodium. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter. How To Determine Osmolality Of A Solution.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 How To Determine Osmolality Of A Solution Osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of water across cell. The osmolarity of solutions containing a single type of solute (for example: An osmole (osmol) is 1 mol of particles that. To calculate the osmolarity of a solution, the first step is to convert the number to moles per. How To Determine Osmolality Of A Solution.
From brainly.com
Osmosis is the movement of ____ across a membrane. a) food b) energy c How To Determine Osmolality Of A Solution The osmolarity of solutions containing a single type of solute (for example: You multiply the molarity by the number of osmoles that each solute produces. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. How do you calculate osmolarity? An osmole is 1 mol of particles that. How To Determine Osmolality Of A Solution.
From www.hanlin.com
CIE A Level Biology复习笔记4.2.9 Estimating Water Potential in Plants翰林国际教育 How To Determine Osmolality Of A Solution To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. How do you calculate osmolarity? Osmolarity is the number of osmoles of solute per litre of solution. Just glucose or just sodium. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d. How To Determine Osmolality Of A Solution.
From www.vrogue.co
Developmental Changes In A Blood Osmolality Osm And B vrogue.co How To Determine Osmolality Of A Solution To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. Osmolarity is the number of osmoles of solute per litre of solution. Indeed, the osmolality of a solution is. How To Determine Osmolality Of A Solution.
From thecontentauthority.com
Molality vs Osmolality How Are These Words Connected? How To Determine Osmolality Of A Solution An osmole is 1 mol of particles that contribute to the. The osmolarity of solutions containing a single type of solute (for example: Convert that number to osmoles per liter and add the osmolarity of each. How do you calculate osmolarity? Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d. How To Determine Osmolality Of A Solution.
From www.reddit.com
How to calculate the osmolality of 0.1 M solution of Na2HPO4 + NaH2PO4 How To Determine Osmolality Of A Solution Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): Osmolarity is the number of osmoles of solute per litre of solution. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. You multiply the molarity by the number of osmoles. How To Determine Osmolality Of A Solution.
From www.youtube.com
How to solve osmolarity calculation problems 3 YouTube How To Determine Osmolality Of A Solution An osmole is 1 mol of particles that contribute to the. Osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of water across cell. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. Just glucose or just sodium. To. How To Determine Osmolality Of A Solution.
From www.slideserve.com
PPT Intravenous Therapy IV Infusion Preparations Fluid and How To Determine Osmolality Of A Solution Osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of water across cell. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. An osmole is 1 mol of particles that contribute to the. Osmolarity is the number of osmoles. How To Determine Osmolality Of A Solution.
From www.numerade.com
SOLVED 6. Determine the molarity and osmolarity of the following How To Determine Osmolality Of A Solution You multiply the molarity by the number of osmoles that each solute produces. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. To calculate the osmolarity in a solution you first need to find the number of osmoles of the solute per liter of. Just glucose or just sodium. Osmolarity. How To Determine Osmolality Of A Solution.
From www.drawittoknowit.com
Active Learning for the Medical Sciences ditki medical & biological How To Determine Osmolality Of A Solution An osmole is 1 mol of particles that contribute to the. How do you calculate osmolarity? Osmolarity is the number of osmoles of solute per litre of solution. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): Osmolarity is a measure of the total concentration of solute particles in. How To Determine Osmolality Of A Solution.
From www.omnicalculator.com
Serum Osmolality Calculator & Formula How To Determine Osmolality Of A Solution The osmolarity of solutions containing a single type of solute (for example: Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): An osmole is 1 mol of particles that contribute to the. Osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement. How To Determine Osmolality Of A Solution.
From www.chegg.com
Solved What is the predicted osmolality of a solution of the How To Determine Osmolality Of A Solution An osmole (osmol) is 1 mol of particles that. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (d tf): Osmolarity is the number of osmoles of solute per litre of solution. Just glucose or just sodium. Osmolarity is a measure of the total concentration of solute particles in a solution,. How To Determine Osmolality Of A Solution.