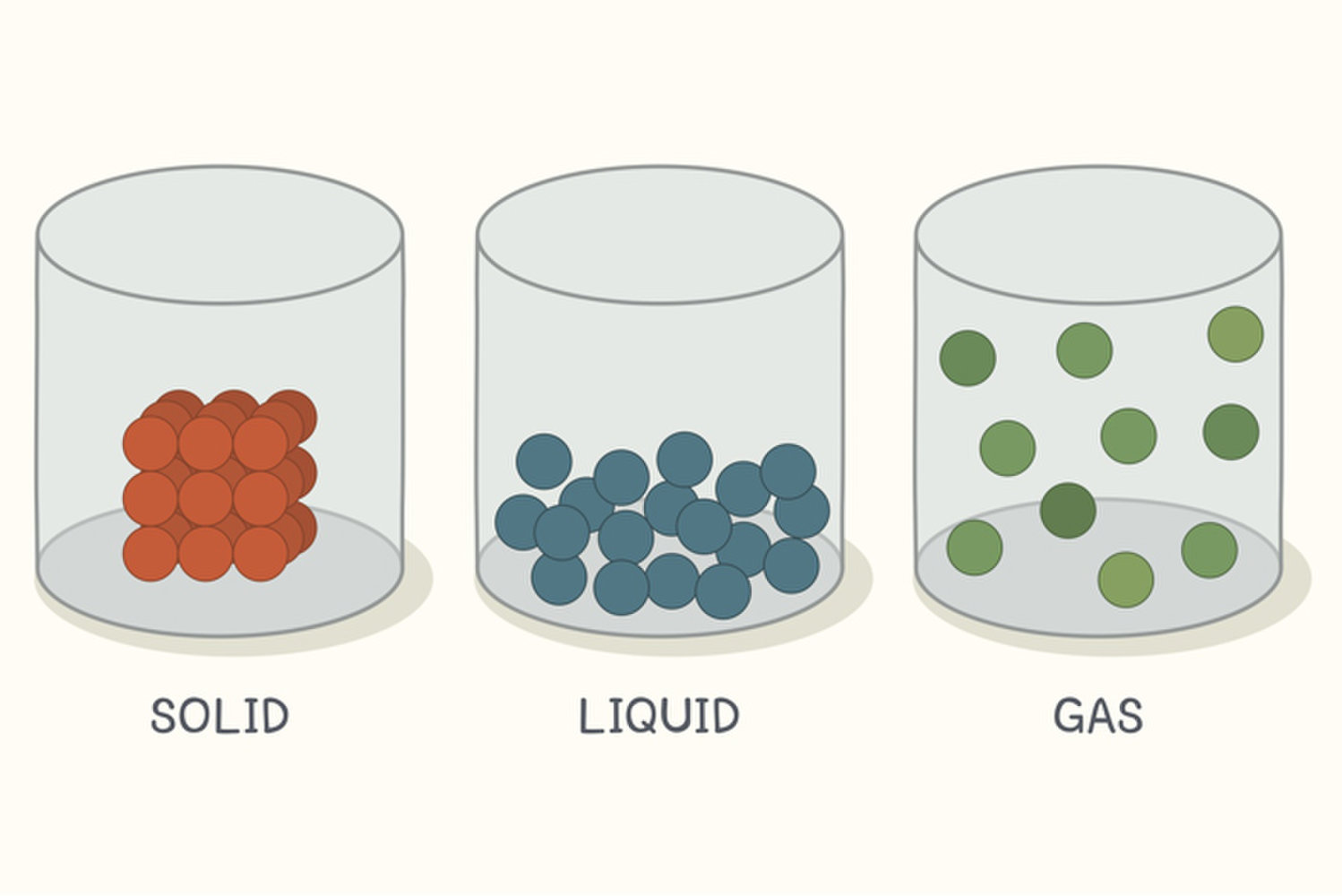
Why Solids And Liquids Hard To Compress . (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space. Gases are very compressible, so when subjected to high. Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. gases have three characteristic properties: The particles in most solids are closely packed together. something is usually described as a solid if it can hold its own shape and is hard to compress (squash). liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. However, it requires a great deal of pressure to accomplish a. the answer is yes, you can compress water, or almost any material. a very important property of a substance is how compressible it is.
from socratic.org
gases have three characteristic properties: (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space. Gases are very compressible, so when subjected to high. the answer is yes, you can compress water, or almost any material. Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. a very important property of a substance is how compressible it is. The particles in most solids are closely packed together. liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. something is usually described as a solid if it can hold its own shape and is hard to compress (squash). However, it requires a great deal of pressure to accomplish a.
What are examples of gases, liquids, and solids? Socratic
Why Solids And Liquids Hard To Compress something is usually described as a solid if it can hold its own shape and is hard to compress (squash). However, it requires a great deal of pressure to accomplish a. liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. Gases are very compressible, so when subjected to high. (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space. The particles in most solids are closely packed together. gases have three characteristic properties: a very important property of a substance is how compressible it is. the answer is yes, you can compress water, or almost any material. Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. something is usually described as a solid if it can hold its own shape and is hard to compress (squash).
From www.britannica.com
phase Definition & Facts Britannica Why Solids And Liquids Hard To Compress Gases are very compressible, so when subjected to high. liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. a very important property of a substance is how compressible it is. the answer is yes, you can compress water, or almost any material. (1) they are easy to compress,. Why Solids And Liquids Hard To Compress.
From chem.libretexts.org
2 The Density of Liquids and Solids (Experiment) Chemistry LibreTexts Why Solids And Liquids Hard To Compress gases have three characteristic properties: The particles in most solids are closely packed together. a very important property of a substance is how compressible it is. something is usually described as a solid if it can hold its own shape and is hard to compress (squash). (1) they are easy to compress, (2) they expand to fill. Why Solids And Liquids Hard To Compress.
From slideplayer.com
IB PHYSICS Topic 3 Thermodynamics ppt download Why Solids And Liquids Hard To Compress the answer is yes, you can compress water, or almost any material. liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. The particles in most solids are closely packed together. However, it requires a great deal of pressure to accomplish a. gases have three characteristic properties: a. Why Solids And Liquids Hard To Compress.
From slideplayer.com
Chapter 14 The Behavior of Gases 14.1 Properties of Gases ppt download Why Solids And Liquids Hard To Compress The particles in most solids are closely packed together. Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. However, it requires a great deal of pressure to accomplish a. liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely. Why Solids And Liquids Hard To Compress.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Why Solids And Liquids Hard To Compress the answer is yes, you can compress water, or almost any material. (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space. Gases are very compressible, so when subjected to high. The particles in most solids are closely packed together. Even though the particles are locked into place and. Why Solids And Liquids Hard To Compress.
From slideplayer.com
P2 Lesson 1 Solids liquids and gases Todays lesson State the Why Solids And Liquids Hard To Compress a very important property of a substance is how compressible it is. the answer is yes, you can compress water, or almost any material. Gases are very compressible, so when subjected to high. However, it requires a great deal of pressure to accomplish a. something is usually described as a solid if it can hold its own. Why Solids And Liquids Hard To Compress.
From socratic.org
What are examples of gases, liquids, and solids? Socratic Why Solids And Liquids Hard To Compress However, it requires a great deal of pressure to accomplish a. gases have three characteristic properties: (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space. the answer is yes, you can compress water, or almost any material. something is usually described as a solid if it. Why Solids And Liquids Hard To Compress.
From quizzaidan123.z19.web.core.windows.net
Science Solid Liquid Gas Why Solids And Liquids Hard To Compress The particles in most solids are closely packed together. However, it requires a great deal of pressure to accomplish a. liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny. Why Solids And Liquids Hard To Compress.
From www.youtube.com
States of matter 🚗💧☁️ Solid, Liquid & Gas Learn with examples YouTube Why Solids And Liquids Hard To Compress a very important property of a substance is how compressible it is. liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. gases have three characteristic properties: The particles in most solids are closely packed together. Gases are very compressible, so when subjected to high. However, it requires a. Why Solids And Liquids Hard To Compress.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Why Solids And Liquids Hard To Compress a very important property of a substance is how compressible it is. liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. something is usually described as a solid if it can hold its own shape and is hard to compress (squash). (1) they are easy to compress, (2). Why Solids And Liquids Hard To Compress.
From chem.libretexts.org
16.2 The Liquid State Chemistry LibreTexts Why Solids And Liquids Hard To Compress Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. Gases are very compressible, so when subjected to high. a very important property of a substance is how compressible it is. (1) they are easy to compress, (2) they expand to fill their containers, and (3) they. Why Solids And Liquids Hard To Compress.
From www.youtube.com
Can solids, liquids and gases be compressed? Grade Three Science Why Solids And Liquids Hard To Compress gases have three characteristic properties: the answer is yes, you can compress water, or almost any material. However, it requires a great deal of pressure to accomplish a. Gases are very compressible, so when subjected to high. The particles in most solids are closely packed together. something is usually described as a solid if it can hold. Why Solids And Liquids Hard To Compress.
From online-learning-college.com
States of matter solids, liquids and gases Interconversions Why Solids And Liquids Hard To Compress However, it requires a great deal of pressure to accomplish a. liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. a very important property of a substance is how compressible it is. The particles in most solids are closely packed together. the answer is yes, you can compress. Why Solids And Liquids Hard To Compress.
From elementary.tvolearn.com
Understanding Density and Compressibility Relationships Why Solids And Liquids Hard To Compress a very important property of a substance is how compressible it is. (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space. gases have three characteristic properties: Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny. Why Solids And Liquids Hard To Compress.
From slideplayer.com
Chapter 11 Intermolecular Forces, Liquids and Solids ppt download Why Solids And Liquids Hard To Compress something is usually described as a solid if it can hold its own shape and is hard to compress (squash). Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and. Why Solids And Liquids Hard To Compress.
From www.sciencefacts.net
Physics Page 7 of 19 Science Facts Why Solids And Liquids Hard To Compress liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. Gases are very compressible, so when subjected to high. The particles in most solids are closely packed together. a very important property of a substance is how compressible it is. Even though the particles are locked into place and cannot. Why Solids And Liquids Hard To Compress.
From exongvijw.blob.core.windows.net
Lesson On Solids Liquids And Gases at Pauline Birdsell blog Why Solids And Liquids Hard To Compress Gases are very compressible, so when subjected to high. The particles in most solids are closely packed together. the answer is yes, you can compress water, or almost any material. liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. gases have three characteristic properties: (1) they are easy. Why Solids And Liquids Hard To Compress.
From courses.lumenlearning.com
8.2 Solids and Liquids The Basics of General, Organic, and Biological Why Solids And Liquids Hard To Compress liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. a very important property of a substance is how compressible it is. gases have three characteristic properties: the answer is yes, you can compress water, or almost any material. However, it requires a great deal of pressure to. Why Solids And Liquids Hard To Compress.
From exosizztp.blob.core.windows.net
Solids Compressibility at Wilson blog Why Solids And Liquids Hard To Compress Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space. something is usually described as a solid if it can hold its own shape and is hard. Why Solids And Liquids Hard To Compress.
From exotghnhb.blob.core.windows.net
Properties Of Solids And Liquids at Susan Villanueva blog Why Solids And Liquids Hard To Compress the answer is yes, you can compress water, or almost any material. something is usually described as a solid if it can hold its own shape and is hard to compress (squash). Gases are very compressible, so when subjected to high. a very important property of a substance is how compressible it is. gases have three. Why Solids And Liquids Hard To Compress.
From diagramlibraryconjoin.z19.web.core.windows.net
Solid Liquid And Gas Diagram Why Solids And Liquids Hard To Compress something is usually described as a solid if it can hold its own shape and is hard to compress (squash). the answer is yes, you can compress water, or almost any material. Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. However, it requires a. Why Solids And Liquids Hard To Compress.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids and Solids PowerPoint Why Solids And Liquids Hard To Compress Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. However, it requires a great deal of pressure to accomplish a. liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. (1) they are easy to compress, (2) they. Why Solids And Liquids Hard To Compress.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Why Solids And Liquids Hard To Compress the answer is yes, you can compress water, or almost any material. liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. However, it requires a great deal of pressure to accomplish a. something is usually described as a solid if it can hold its own shape and is. Why Solids And Liquids Hard To Compress.
From exotuafat.blob.core.windows.net
Liquid Vs Solid at James Foerster blog Why Solids And Liquids Hard To Compress (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space. something is usually described as a solid if it can hold its own shape and is hard to compress (squash). a very important property of a substance is how compressible it is. the answer is yes, you. Why Solids And Liquids Hard To Compress.
From www.learnatnoon.com
The Molecular Differences Between Solids, Liquid and Gases Why Solids And Liquids Hard To Compress gases have three characteristic properties: something is usually described as a solid if it can hold its own shape and is hard to compress (squash). liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. a very important property of a substance is how compressible it is. However,. Why Solids And Liquids Hard To Compress.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Why Solids And Liquids Hard To Compress The particles in most solids are closely packed together. Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. something is usually described as a solid if it can hold its own shape and is hard to compress (squash). (1) they are easy to compress, (2) they. Why Solids And Liquids Hard To Compress.
From www.britannica.com
solid Definition & Facts Britannica Why Solids And Liquids Hard To Compress The particles in most solids are closely packed together. gases have three characteristic properties: the answer is yes, you can compress water, or almost any material. something is usually described as a solid if it can hold its own shape and is hard to compress (squash). However, it requires a great deal of pressure to accomplish a.. Why Solids And Liquids Hard To Compress.
From sciencenotes.org
Liquid Definition Examples of Liquids Why Solids And Liquids Hard To Compress Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. gases have three characteristic properties: The particles in most solids are closely packed together. Gases are very compressible, so when subjected to high. However, it requires a great deal of pressure to accomplish a. the answer. Why Solids And Liquids Hard To Compress.
From slideplayer.com
Solids Liquids Gases TB p ppt download Why Solids And Liquids Hard To Compress liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. Gases are very compressible, so when subjected to high. Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. The particles in most solids are closely packed together. (1). Why Solids And Liquids Hard To Compress.
From blog.mensor.com
What Exactly is The Compressibility of Fluids? Why Solids And Liquids Hard To Compress the answer is yes, you can compress water, or almost any material. gases have three characteristic properties: Gases are very compressible, so when subjected to high. Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. something is usually described as a solid if it. Why Solids And Liquids Hard To Compress.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Why Solids And Liquids Hard To Compress The particles in most solids are closely packed together. However, it requires a great deal of pressure to accomplish a. Gases are very compressible, so when subjected to high. Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. a very important property of a substance is. Why Solids And Liquids Hard To Compress.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Why Solids And Liquids Hard To Compress liquids will be compressed, resulting in lots of heat as this happens (with infinite pressure, and infinitely strong. gases have three characteristic properties: Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. (1) they are easy to compress, (2) they expand to fill their containers,. Why Solids And Liquids Hard To Compress.
From www.slideserve.com
PPT Unit 3 Fluids & Dynamics PowerPoint Presentation, free download Why Solids And Liquids Hard To Compress something is usually described as a solid if it can hold its own shape and is hard to compress (squash). (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space. a very important property of a substance is how compressible it is. gases have three characteristic properties:. Why Solids And Liquids Hard To Compress.
From slideplayer.com
By Autumn, Emma, Helen, Sophia and Tamarah ppt download Why Solids And Liquids Hard To Compress Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. The particles in most solids are closely packed together. a very important property of a substance is how compressible it is. Gases are very compressible, so when subjected to high. something is usually described as a. Why Solids And Liquids Hard To Compress.
From slideplayer.com
Physical Properties of Matter ppt download Why Solids And Liquids Hard To Compress (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space. However, it requires a great deal of pressure to accomplish a. a very important property of a substance is how compressible it is. the answer is yes, you can compress water, or almost any material. gases have. Why Solids And Liquids Hard To Compress.