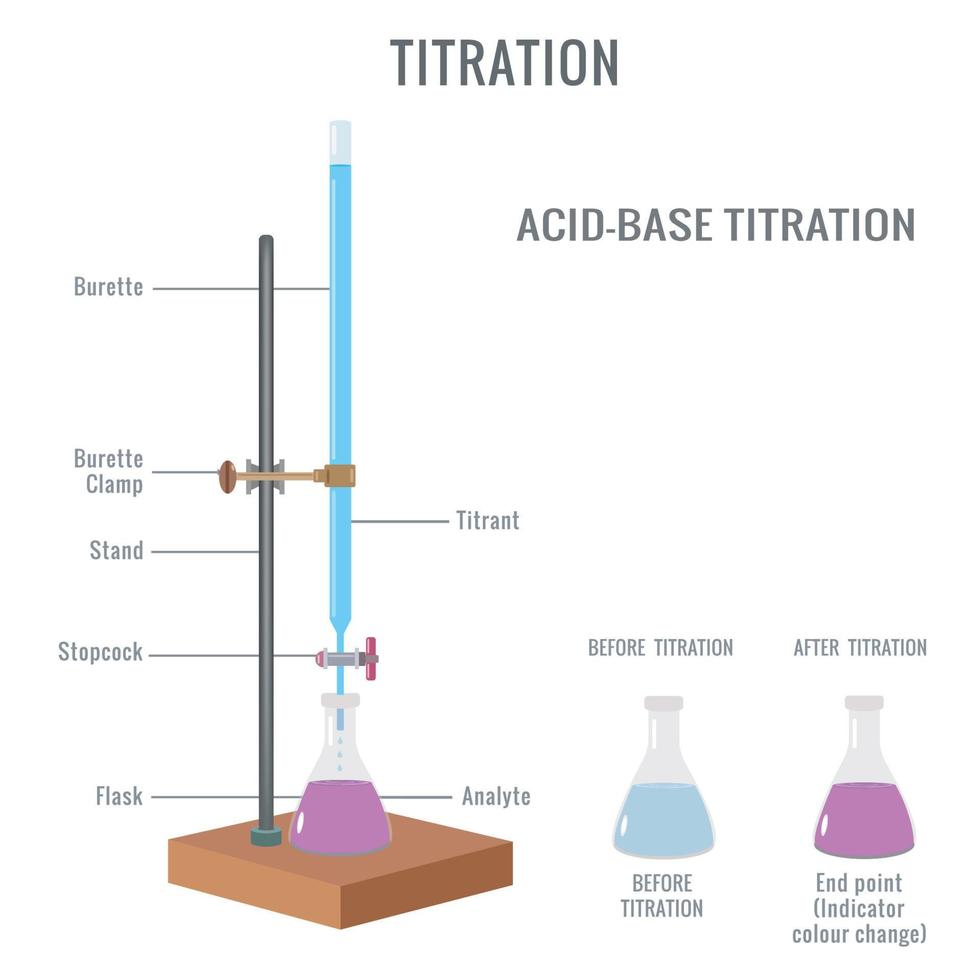
Titration Experiment Class 11 . • experiment 5.1 :to determine the ph of some fruit juices • experiment 5.2 : Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution of oxalic acid. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Follow the procedure, observations, calculations and. Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data.
from www.vecteezy.com
Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Follow the procedure, observations, calculations and. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. • experiment 5.1 :to determine the ph of some fruit juices • experiment 5.2 : Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution of oxalic acid.
Acid base titration experiment and phases of color change during
Titration Experiment Class 11 Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution of oxalic acid. To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Follow the procedure, observations, calculations and. Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. • experiment 5.1 :to determine the ph of some fruit juices • experiment 5.2 : Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution of oxalic acid.
From www.studypool.com
SOLUTION Titration Chemistry Class 11th Studypool Titration Experiment Class 11 Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. Follow the procedure, observations, calculations and. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution. Titration Experiment Class 11.
From www.youtube.com
DSE04 (13) Titration calculation through simulation YouTube Titration Experiment Class 11 Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. • experiment 5.1 :to determine. Titration Experiment Class 11.
From boardtopper.blogspot.com
Titration 2 Class 12 CBSE Practical All Study Guide at one Place! Titration Experiment Class 11 Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Follow the procedure, observations, calculations and. • experiment 5.1 :to determine the ph of some fruit juices • experiment 5.2 : Now we will learn about the titration practical for titration of oxalic acid for the estimation of. Titration Experiment Class 11.
From loeayhifn.blob.core.windows.net
Titration Higher Chemistry at Clarence Reeves blog Titration Experiment Class 11 Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Learn how. Titration Experiment Class 11.
From www.tes.com
Acid Base Titration Theory, Titration Lab, Titration Stoichiometry Titration Experiment Class 11 Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution of oxalic acid. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Follow the procedure, observations, calculations and. Learn how to determine the concentration of potassium permanganate solution by. Titration Experiment Class 11.
From www.youtube.com
What is Titration in Chemistry Chemistry practical Class 12th Titration Experiment Class 11 • experiment 5.1 :to determine the ph of some fruit juices • experiment 5.2 : Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Follow the procedure, observations, calculations and.. Titration Experiment Class 11.
From www.youtube.com
Chemistry PracticalTitration ExperimentAcid Base Titration Theory Titration Experiment Class 11 To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution of oxalic acid. • experiment 5.1. Titration Experiment Class 11.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Experiment Class 11 To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. • experiment 5.1 :to determine the ph of some fruit juices • experiment 5.2 : Follow the procedure, observations, calculations and. Learn how to. Titration Experiment Class 11.
From joizlgxlm.blob.core.windows.net
Titration Experiments In Supramolecular Chemistry at Larry Ferrill blog Titration Experiment Class 11 Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution of oxalic acid. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. Follow the procedure,. Titration Experiment Class 11.
From mungfali.com
Titration Steps Titration Experiment Class 11 Follow the procedure, observations, calculations and. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. Learn how to determine. Titration Experiment Class 11.
From studylib.net
Experiment 6 EDTA Titration of the Hardness of Water Titration Experiment Class 11 • experiment 5.1 :to determine the ph of some fruit juices • experiment 5.2 : Follow the procedure, observations, calculations and. Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Learn how to. Titration Experiment Class 11.
From www.scribd.com
Titration Class XI PDF Titration Experiment Class 11 Follow the procedure, observations, calculations and. Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution of oxalic acid. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. To observe the variation in ph of acid/base with dilution • experiment 5.3 :to.. Titration Experiment Class 11.
From boardtopper.blogspot.com
Titration 1 Class 12 CBSE Chemistry Practical All Study Guide at Titration Experiment Class 11 To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. Learn how to determine the molarity of a. Titration Experiment Class 11.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Experiment Class 11 • experiment 5.1 :to determine the ph of some fruit juices • experiment 5.2 : Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution of oxalic acid. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Learn about titrimetric analysis, a method of. Titration Experiment Class 11.
From byjus.com
Study The pH Change In The Titration Of A Strong Base Using Universal Titration Experiment Class 11 Follow the procedure, observations, calculations and. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. • experiment. Titration Experiment Class 11.
From www.studocu.com
Class XII Chemistry 202223 Experiment No Oxidation Reduction Titration Experiment Class 11 Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution of oxalic acid. • experiment 5.1 :to determine the ph of some fruit juices • experiment 5.2 : Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Learn about titrimetric analysis, a method of. Titration Experiment Class 11.
From joieldmit.blob.core.windows.net
How Is Titration Used In Medicine at Shelia Wiley blog Titration Experiment Class 11 Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. • experiment 5.1 :to determine the ph of some fruit juices • experiment 5.2 : Learn how to determine the molarity. Titration Experiment Class 11.
From ubicaciondepersonas.cdmx.gob.mx
Titration Equipment Kit ubicaciondepersonas.cdmx.gob.mx Titration Experiment Class 11 Follow the procedure, observations, calculations and. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Now we will learn about the titration practical for titration of oxalic acid for the. Titration Experiment Class 11.
From www.youtube.com
️ Titration Experiment for Board Class Complete Video to Understand Titration Experiment Class 11 Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Follow the procedure, observations, calculations and. Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. Now we will learn about the titration practical for titration of oxalic acid. Titration Experiment Class 11.
From www.youtube.com
CLASS XI TOPIC TITRATION OXALIC ACID AND SODIUM HYDROXIDE Titration Experiment Class 11 Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Learn how. Titration Experiment Class 11.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Titration Experiment Class 11 Follow the procedure, observations, calculations and. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. To observe the variation in ph of acid/base with dilution • experiment 5.3. Titration Experiment Class 11.
From boardtopper.blogspot.com
Titration 1 Class 12 CBSE Chemistry Practical All Study Guide at Titration Experiment Class 11 Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution of oxalic acid. To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Learn how to. Titration Experiment Class 11.
From www.alamy.com
Titration experiment, adding chemical of known concentration to test Titration Experiment Class 11 To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Follow the procedure, observations, calculations and. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution. Titration Experiment Class 11.
From www.youtube.com
KMnO4 VS Mohr’s Salt Titration, Class 12 by Kanchan Handa YouTube Titration Experiment Class 11 Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Follow the procedure, observations, calculations and. • experiment 5.1 :to determine the ph of some fruit juices • experiment. Titration Experiment Class 11.
From www.alamy.com
Titration experiment to measure the volume of acid needed to neutralize Titration Experiment Class 11 Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Follow the procedure, observations, calculations and. Learn how to determine. Titration Experiment Class 11.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Experiment Class 11 Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution of. Titration Experiment Class 11.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Experiment Class 11 To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. Follow the procedure, observations, calculations and. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction. Titration Experiment Class 11.
From www.chegg.com
Solved QUESTION 1 A grade 12 learner performed a titration Titration Experiment Class 11 Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. • experiment 5.1 :to determine. Titration Experiment Class 11.
From www.pdfprof.com
calculate the molarity of 1.60 l of a solution Titration Experiment Class 11 To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. Follow the procedure, observations, calculations and. Learn about titrimetric analysis,. Titration Experiment Class 11.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration Experiment Class 11 To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. • experiment 5.1 :to determine the ph. Titration Experiment Class 11.
From joixjswcw.blob.core.windows.net
Titration Of Acid Base Experiment at Joanne Rivera blog Titration Experiment Class 11 Learn how to determine the molarity of a sodium hydroxide solution by titrating it against a standard solution of oxalic acid. • experiment 5.1 :to determine the ph of some fruit juices • experiment 5.2 : Follow the procedure, observations, calculations and. Find out how to select a suitable indicator and calculate ph and pka/pkb values from titration data. To. Titration Experiment Class 11.
From mavink.com
Sodium Hydroxide Ph Chart Titration Experiment Class 11 • experiment 5.1 :to determine the ph of some fruit juices • experiment 5.2 : Follow the procedure, observations, calculations and. Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in. Titration Experiment Class 11.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Experiment Class 11 Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Follow the procedure, observations, calculations and. • experiment 5.1 :to determine the ph of some fruit juices • experiment 5.2 : Learn how to determine the molarity. Titration Experiment Class 11.
From www.studocu.com
Titration GRADE 12 PRESCRIBED EXPERIMENT 2 ACIDBASE REACTIONS WORK Titration Experiment Class 11 Learn about titrimetric analysis, a method of determining the amount of substance in solution by adding standard solution until the reaction is. Follow the procedure, observations, calculations and. Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. To observe the variation in ph of acid/base with dilution • experiment 5.3. Titration Experiment Class 11.
From klaqnshvp.blob.core.windows.net
How To Do A Titration Rsc at Paul Gallegos blog Titration Experiment Class 11 To observe the variation in ph of acid/base with dilution • experiment 5.3 :to. Now we will learn about the titration practical for titration of oxalic acid for the estimation of sodium hydroxide. Learn how to determine the concentration of potassium permanganate solution by titrating it against oxalic acid in acidic medium. Learn how to determine the molarity of a. Titration Experiment Class 11.