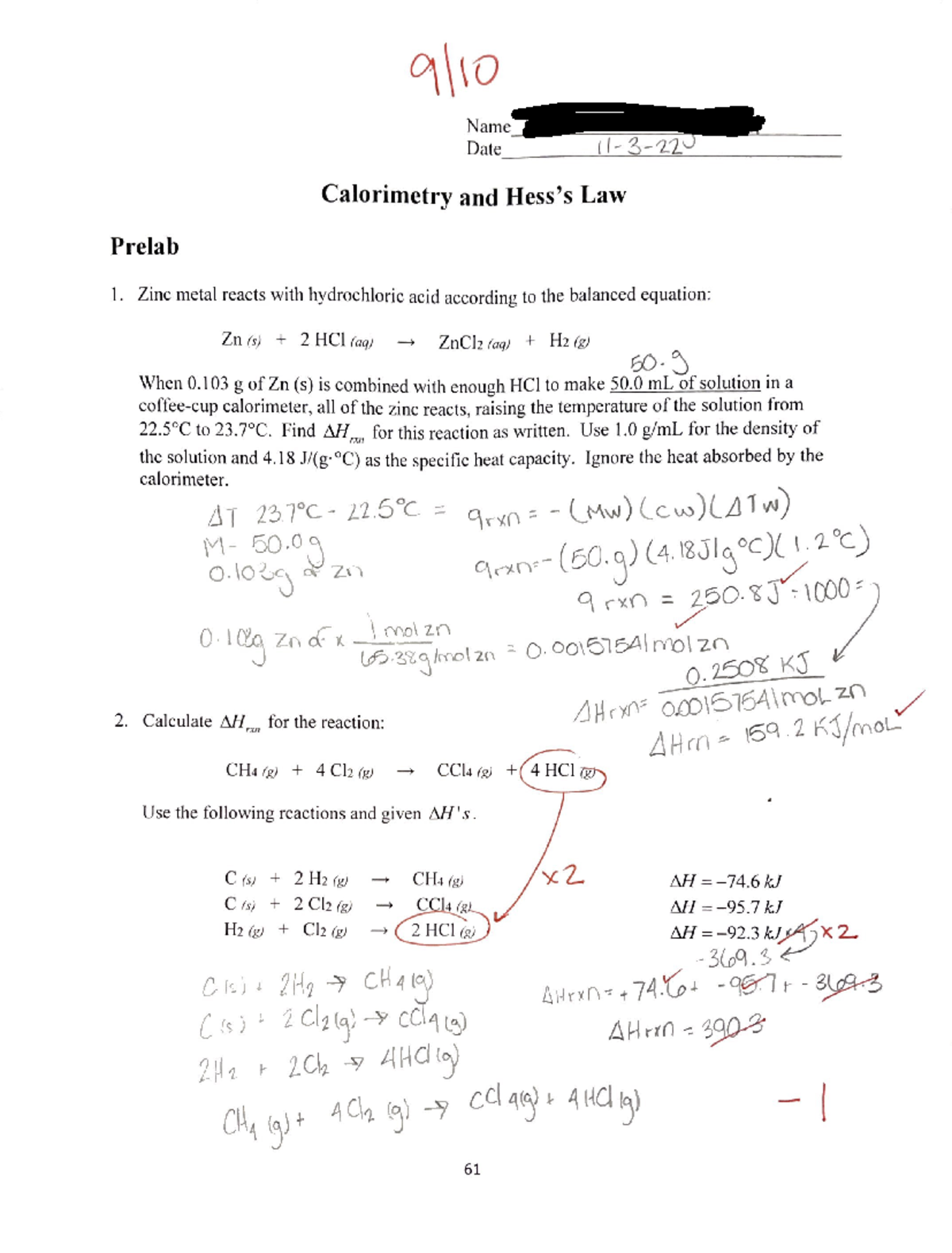
Calorimetry And Hess's Law Lab . To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. The concept of a state function. Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. Calorimetry is the technique used to measure the heat required or evolved during a chemical. Calorimetry and hess’s law, online chemistry lab manual. Hess’s law states that if a reaction can be expressed as the sum of two other reactions,. Calorimetery and hess’s law overview: Calorimetry and hess’s law objectives use calorimetric measurements to determine heats of reaction and to demonstrate hess’s law of. In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Calorimetry is the measurement of the heat change for a reaction, and the device used to measure heat changes is a calorimeter. To apply these δh values in a hess’s law calculation to determine the. Demonstrates the concept of a state function.
from www.studocu.com
Hess’s law states that if a reaction can be expressed as the sum of two other reactions,. Demonstrates the concept of a state function. Calorimetry and hess’s law objectives use calorimetric measurements to determine heats of reaction and to demonstrate hess’s law of. Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. Calorimetery and hess’s law overview: In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Calorimetry and hess’s law, online chemistry lab manual. To apply these δh values in a hess’s law calculation to determine the. The concept of a state function. Calorimetry is the technique used to measure the heat required or evolved during a chemical.
Calorimetry and Hess law prelab CHM 2210C Studocu
Calorimetry And Hess's Law Lab Hess’s law states that if a reaction can be expressed as the sum of two other reactions,. Calorimetry is the measurement of the heat change for a reaction, and the device used to measure heat changes is a calorimeter. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Hess’s law states that if a reaction can be expressed as the sum of two other reactions,. The concept of a state function. To apply these δh values in a hess’s law calculation to determine the. In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Calorimetery and hess’s law overview: Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. Demonstrates the concept of a state function. Calorimetry is the technique used to measure the heat required or evolved during a chemical. Calorimetry and hess’s law objectives use calorimetric measurements to determine heats of reaction and to demonstrate hess’s law of. Calorimetry and hess’s law, online chemistry lab manual.
From www.numerade.com
SOLVED Calorimetry and Hess' Law Report; Submit = your data Blackboard Calorimetry And Hess's Law Lab Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. Hess’s law states that if a reaction can be expressed as the sum of two other reactions,. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Calorimetry and hess’s law objectives use. Calorimetry And Hess's Law Lab.
From www.chegg.com
Lab Report Calorimetry and Hess's Law Metal + HCI Calorimetry And Hess's Law Lab Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. The concept of a state function. Calorimetry is the measurement of the heat change for a reaction, and the device used to measure heat changes is a calorimeter. To experimentally measure the δh values of two reactions using the. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved ENTHALPY OF NEUTRALIZATION AND HESS LAW Post Calorimetry And Hess's Law Lab Calorimetry is the measurement of the heat change for a reaction, and the device used to measure heat changes is a calorimeter. Hess’s law states that if a reaction can be expressed as the sum of two other reactions,. Calorimetry and hess’s law objectives use calorimetric measurements to determine heats of reaction and to demonstrate hess’s law of. Calorimetery and. Calorimetry And Hess's Law Lab.
From www.chegg.com
Thermochemistry Calorimetry and Hess's Law Date Calorimetry And Hess's Law Lab In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Hess’s law states that if a reaction can be expressed as the sum of two other reactions,. Calorimetry and hess’s law objectives use calorimetric measurements to determine heats of reaction and to demonstrate. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved Name Calorimetry Hess's Law Drawer Number PreLab Calorimetry And Hess's Law Lab Demonstrates the concept of a state function. In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. The concept of a state function. Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy. Calorimetry And Hess's Law Lab.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Calorimetry And Hess's Law Lab To apply these δh values in a hess’s law calculation to determine the. Calorimetry is the measurement of the heat change for a reaction, and the device used to measure heat changes is a calorimeter. Calorimetry and hess’s law, online chemistry lab manual. Demonstrates the concept of a state function. Calorimetry is the technique used to measure the heat required. Calorimetry And Hess's Law Lab.
From www.studocu.com
Exp 9 Calorimetry and Hess’ Law Lab work NAME Diana Koshy Valliath Calorimetry And Hess's Law Lab Calorimetry is the technique used to measure the heat required or evolved during a chemical. In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Calorimetery and hess’s law overview: Calorimetry and hess’s law objectives use calorimetric measurements to determine heats of reaction. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved Prelab Assignment Calorimetry and Hess's Law Show Calorimetry And Hess's Law Lab Calorimetry is the technique used to measure the heat required or evolved during a chemical. To apply these δh values in a hess’s law calculation to determine the. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. In order to use hess’s law to find the heat of combustion of a metal, it. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved Lab Report Calorimetry and Hess's Law Metal + HCI Calorimetry And Hess's Law Lab The concept of a state function. Calorimetery and hess’s law overview: Calorimetry and hess’s law, online chemistry lab manual. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Calorimetry is the technique used to measure the heat required or evolved during a chemical. Demonstrates the concept of a state function. In order to. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved Lab Report Calorimetry and Hess's Law Metal + HCI Calorimetry And Hess's Law Lab In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Calorimetry is the measurement of the heat change for a reaction, and the device used to measure heat changes is a calorimeter. Hess’s law states that if a reaction can be expressed as. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved Experiment 12 Calorimetry and Hess' Law Calorimetry And Hess's Law Lab In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Calorimetery and hess’s law overview: To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Calorimetry and hess’s law, online chemistry lab manual. To apply these δh. Calorimetry And Hess's Law Lab.
From www.studocu.com
Calorimetry and Hess law prelab CHM 2210C Studocu Calorimetry And Hess's Law Lab Hess’s law states that if a reaction can be expressed as the sum of two other reactions,. In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Calorimetry is the technique used to measure the heat required or evolved during a chemical. Calorimetry. Calorimetry And Hess's Law Lab.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Calorimetry And Hess's Law Lab To apply these δh values in a hess’s law calculation to determine the. Calorimetry is the measurement of the heat change for a reaction, and the device used to measure heat changes is a calorimeter. Calorimetry is the technique used to measure the heat required or evolved during a chemical. Demonstrates the concept of a state function. The concept of. Calorimetry And Hess's Law Lab.
From www.studocu.com
Lab 4 Styrofoam Cup Calorimetry and Hess’s Law Lab Report Table A Calorimetry And Hess's Law Lab To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Calorimetry is the measurement of the heat change for a reaction, and the device used to measure heat changes is a calorimeter. In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction. Calorimetry And Hess's Law Lab.
From www.chegg.com
25 Name Experiment 2 Calorimetry Hess's Law PreLab Calorimetry And Hess's Law Lab Calorimetry and hess’s law objectives use calorimetric measurements to determine heats of reaction and to demonstrate hess’s law of. Calorimetry is the technique used to measure the heat required or evolved during a chemical. Demonstrates the concept of a state function. Calorimetry and hess’s law, online chemistry lab manual. The concept of a state function. To experimentally measure the δh. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved Experiment 7 Calorimetry and Hess's Law Pre Lab Calorimetry And Hess's Law Lab Demonstrates the concept of a state function. Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. Calorimetry and hess’s law objectives use calorimetric measurements to determine heats of reaction and to demonstrate hess’s law of. Calorimetry is the technique used to measure the heat required or evolved during. Calorimetry And Hess's Law Lab.
From www.studocu.com
Lab Manual Experiment 1 Calorimetry HESS'S LAW Applied Chemistry Calorimetry And Hess's Law Lab Demonstrates the concept of a state function. Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. Calorimetry and hess’s law, online chemistry lab manual. Calorimetry and hess’s law objectives use calorimetric measurements to determine heats of reaction and to demonstrate hess’s law of. Hess’s law states that if. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved Name Calorimetry Hess's Law Drawer Number PreLab Calorimetry And Hess's Law Lab To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Calorimetry is the technique used to measure the heat required or evolved during a chemical. Hess’s law states that if a reaction can be expressed as the sum of two other reactions,. To apply these δh values in a hess’s law calculation to determine. Calorimetry And Hess's Law Lab.
From www.flinnsci.com
Heats of Reaction and Hess’s Law SmallScale Calorimetry—ChemTopic Calorimetry And Hess's Law Lab Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. Calorimetry is the technique used to measure the heat required or evolved during a chemical. Calorimetry is the measurement of the heat change for a reaction, and the device used to measure heat changes is a calorimeter. To experimentally. Calorimetry And Hess's Law Lab.
From schoolworkhelper.net
Hess’ Law & Calorimetry Lab Explained SchoolWorkHelper Calorimetry And Hess's Law Lab Demonstrates the concept of a state function. Calorimetry and hess’s law objectives use calorimetric measurements to determine heats of reaction and to demonstrate hess’s law of. Hess’s law states that if a reaction can be expressed as the sum of two other reactions,. To apply these δh values in a hess’s law calculation to determine the. To experimentally measure the. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved Lab Report Calorimetry and Hess's Law 24.7089 Metal Calorimetry And Hess's Law Lab Calorimetry is the technique used to measure the heat required or evolved during a chemical. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Calorimetry is the measurement of the heat change for a reaction, and the device used to measure heat changes is a calorimeter. The concept of a state function. Calorimetry. Calorimetry And Hess's Law Lab.
From lazaro.perka.org
Calorimetry And Hess'S Law Pre Lab Answers lazaro Calorimetry And Hess's Law Lab Calorimetry and hess’s law, online chemistry lab manual. Calorimetry and hess’s law objectives use calorimetric measurements to determine heats of reaction and to demonstrate hess’s law of. Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. In order to use hess’s law to find the heat of combustion. Calorimetry And Hess's Law Lab.
From www.chegg.com
Lab Report Calorimetry and Hess's Law Metal + HCI Calorimetry And Hess's Law Lab Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. Demonstrates the concept of a state function. In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Calorimetery and hess’s law overview:. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved Date Name Lab Section Prelab Assignment Calorimetry And Hess's Law Lab In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. Calorimetry and hess’s law, online chemistry lab manual. Demonstrates the concept of. Calorimetry And Hess's Law Lab.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Calorimetry And Hess's Law Lab Hess’s law states that if a reaction can be expressed as the sum of two other reactions,. In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Calorimetry and hess’s law, online chemistry lab manual. The concept of a state function. Calorimetry is. Calorimetry And Hess's Law Lab.
From www.docsity.com
Calorimetry and Hess's Law in Chemistry Lab Experiment 3 CHEM 1 Calorimetry And Hess's Law Lab Calorimetry and hess’s law, online chemistry lab manual. Calorimetry and hess’s law objectives use calorimetric measurements to determine heats of reaction and to demonstrate hess’s law of. The concept of a state function. To apply these δh values in a hess’s law calculation to determine the. Hess’s law states that if a reaction can be expressed as the sum of. Calorimetry And Hess's Law Lab.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry And Hess's Law Lab Calorimetry is the technique used to measure the heat required or evolved during a chemical. In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then. Calorimetry And Hess's Law Lab.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry And Hess's Law Lab To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Hess’s law states that if a reaction can be expressed as the sum of two other reactions,. Calorimetry is the technique used to measure the heat required or evolved during a chemical. Calorimetery and hess’s law overview: To apply these δh values in a. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved Thermochemistry Calorimetry and Hess's Law Name Calorimetry And Hess's Law Lab Demonstrates the concept of a state function. The concept of a state function. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Calorimetry and hess’s law objectives use calorimetric measurements to determine heats of reaction and to demonstrate hess’s law of. Hess’s law states that if a reaction can be expressed as the. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved Lab 8 Calorimetry and Hess's Law As you have seen, Calorimetry And Hess's Law Lab In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. To experimentally measure the δh values of two reactions using the technique. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved Lab Report Calorimetry and Hess's Law Metal + HCI Calorimetry And Hess's Law Lab The concept of a state function. Calorimetry is the measurement of the heat change for a reaction, and the device used to measure heat changes is a calorimeter. Demonstrates the concept of a state function. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. To apply these δh values in a hess’s law. Calorimetry And Hess's Law Lab.
From www.youtube.com
Thermochemistry Calorimetry and Hess's Law YouTube Calorimetry And Hess's Law Lab The concept of a state function. Calorimetry is the technique used to measure the heat required or evolved during a chemical. Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. To apply these δh values in a hess’s law calculation to determine the. Calorimetry is the measurement of. Calorimetry And Hess's Law Lab.
From www.chegg.com
Solved Lab Report Calorimetry and Hess's Law Metal + HCI Calorimetry And Hess's Law Lab Calorimetry is the measurement of the heat change for a reaction, and the device used to measure heat changes is a calorimeter. Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. The concept of a state function. Calorimetry is the technique used to measure the heat required or. Calorimetry And Hess's Law Lab.
From www.docsity.com
Calorimetry and Hess’s Law Lab Report Lab Reports Chemistry Docsity Calorimetry And Hess's Law Lab To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Calorimetery and hess’s law overview: Demonstrates the concept of a state function. The concept of a state function. Calorimetry is the measurement of the heat change for a reaction, and the device used to measure heat changes is a calorimeter. Calorimetry and hess’s law. Calorimetry And Hess's Law Lab.
From www.studypool.com
SOLUTION Energy, Thermodynamics, Enthalpy, Hess's Law, and Calorimetry Calorimetry And Hess's Law Lab Calorimetery and hess’s law overview: The concept of a state function. In order to use hess’s law to find the heat of combustion of a metal, it is first necessary to find reaction enthalpies (∆h values) for equations. Hess’s law states that if a reaction can be expressed as the sum of two other reactions, then the enthalpy change. Demonstrates. Calorimetry And Hess's Law Lab.